Page 274 - Essentials of physical chemistry
P. 274
236 Essentials of Physical Chemistry
V(x)
box
C
C
C
C
V (x)
π
O X L
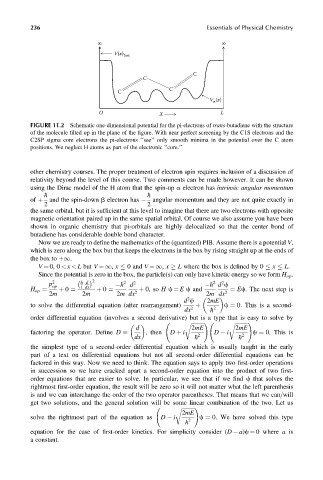
FIGURE 11.2 Schematic one-dimensional potential for the pi-electrons of trans-butadiene with the structure
of the molecule tilted up in the plane of the figure. With near perfect screening by the C1S electrons and the
C2SP sigma core electrons the pi-electrons ‘‘see’’ only smooth minima in the potential over the C atom
positions. We neglect H atoms as part of the electronic ‘‘core.’’
other chemistry courses. The proper treatment of electron spin requires inclusion of a discussion of
relativity beyond the level of this course. Two comments can be made however. It can be shown
using the Dirac model of the H atom that the spin-up a electron has intrinsic angular momentum
h h
of þ and the spin-down b electron has angular momentum and they are not quite exactly in
2 2
the same orbital, but it is sufficient at this level to imagine that there are two electrons with opposite
magnetic orientation paired up in the same spatial orbital. Of course we also assume you have been
shown in organic chemistry that pi-orbitals are highly delocalized so that the center bond of
butadiene has considerable double bond character.
Now we are ready to define the mathematics of the (quantized) PIB. Assume there is a potential V,
which is zero along the box but that keeps the electrons in the box by rising straight up at the ends of
the box to þ1.
V ¼ 0, 0 < x < L but V ¼1, x 0 and V ¼1, x L where the box is defined by 0 x L.
Since the potential is zero in the box, the particle(s) can only have kinetic energy so we form H op .
p 2 h d 2 d 2 d c
2
2
2
h
h
op i dx
H op ¼ þ 0 ¼ þ 0 ¼ 2 þ 0, so H c ¼ E c and 2 ¼ Ec. The next step is
2m 2m 2m dx 2m dx
2
d c 2mE
to solve the differential equation (after rearrangement) þ c ¼ 0. This is a second-
dx 2 h 2
order differential equation (involves a second derivative) but is a type that is easy to solve by
! !
r ffiffiffiffiffiffiffiffiffi r ffiffiffiffiffiffiffiffiffi
d 2mE 2mE
, then D þ i D i c ¼ 0. This is
dx h h
factoring the operator. Define D 2 2
the simplest type of a second-order differential equation which is usually taught in the early
part of a text on differential equations but not all second-order differential equations can be
factored in this way. Now we need to think. The equation says to apply two first-order operations
in succession so we have cracked apart a second-order equation into the product of two first-
order equations that are easier to solve. In particular, we see that if we find c that solves the
rightmost first-order equation, the result will be zero so it will not matter what the left parenthesis
is and we can interchange the order of the two operator parentheses. That means that we can=will
get two solutions, and the general solution will be some linear combination of the two. Let us
!
r ffiffiffiffiffiffiffiffiffi
2mE
solve the rightmost part of the equation as D i 2 c ¼ 0. We have solved this type
h
equation for the case of first-order kinetics. For simplicity consider (D a)c ¼ 0 where a is
a constant.