Page 58 - Essentials of physical chemistry
P. 58
20 Essentials of Physical Chemistry
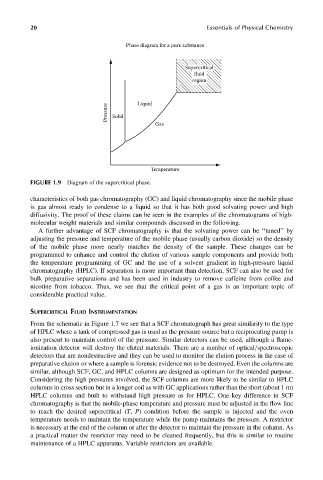
Phase diagram for a pure substance
Supercritical
fluid
region
Pressure Solid Liquid
Gas
Temperature
FIGURE 1.9 Diagram of the supercritical phase.
characteristics of both gas chromatography (GC) and liquid chromatography since the mobile phase
is gas almost ready to condense to a liquid so that it has both good solvating power and high
diffusivity. The proof of these claims can be seen in the examples of the chromatograms of high-
molecular weight materials and similar compounds discussed in the following.
A further advantage of SCF chromatography is that the solvating power can be ‘‘tuned’’ by
adjusting the pressure and temperature of the mobile phase (usually carbon dioxide) so the density
of the mobile phase more nearly matches the density of the sample. These changes can be
programmed to enhance and control the elution of various sample components and provide both
the temperature programming of GC and the use of a solvent gradient in high-pressure liquid
chromatography (HPLC). If separation is more important than detection, SCF can also be used for
bulk preparative separations and has been used in industry to remove caffeine from coffee and
nicotine from tobacco. Thus, we see that the critical point of a gas is an important topic of
considerable practical value.
SUPERCRITICAL FLUID INSTRUMENTATION
From the schematic in Figure 1.7 we see that a SCF chromatograph has great similarity to the type
of HPLC where a tank of compressed gas is used as the pressure source but a reciprocating pump is
also present to maintain control of the pressure. Similar detectors can be used, although a flame-
ionization detector will destroy the eluted materials. There are a number of optical=spectroscopic
detectors that are nondestructive and they can be used to monitor the elution process in the case of
preparative elution or where a sample is forensic evidence not to be destroyed. Even the columns are
similar, although SCF, GC, and HPLC columns are designed as optimum for the intended purpose.
Considering the high pressures involved, the SCF columns are more likely to be similar to HPLC
columns in cross section but in a longer coil as with GC applications rather than the short (about 1 m)
HPLC columns and built to withstand high pressure as for HPLC. One key difference in SCF
chromatography is that the mobile-phase temperature and pressure must be adjusted in the flow line
to reach the desired supercritical (T, P) condition before the sample is injected and the oven
temperature needs to maintain the temperature while the pump maintains the pressure. A restrictor
is necessary at the end of the column or after the detector to maintain the pressure in the column. As
a practical matter the restrictor may need to be cleaned frequently, but this is similar to routine
maintenance of a HPLC apparatus. Variable restrictors are available.