Page 59 - Essentials of physical chemistry
P. 59
Ideal and Real Gas Behavior 21
SUPERCRITICAL MOBILE PHASE
While it is possible to use a number of volatile solvents as the mobile phase for SCF chromatog-
raphy, the most commonly used mobile phase is carbon dioxide. However, carbon dioxide is not a
good solvent for polar compounds so it is common to add a small amount of some additional polar
organic liquid such as an alcohol or even water as a ‘‘modifier.’’ However, the modifier needs to be
miscible with carbon dioxide. Much of the other technology associated with either GC or HPLC in
terms of sample inlets and types of pumps are adapted to specific applications but the key attribute
of the SCF-type chromatography is the maintenance of (T, P) conditions near the critical point of the
mobile phase. A selection of columns is available just as for GC or HPLC.
SAMPLE SCF SEPARATIONS
The output results of an SCF chromatogram are presented on a strip chart recorder showing the
detector response on the vertical axis and the elution time on the horizontal axis. We show two
examples as presented by Karey O’Leary at Virginia Tech University in 1995 (results shown are by
permission from http:==www.cee.vt.edu=ewr=environmental=teach=smprimer=sfc=sfc.html).
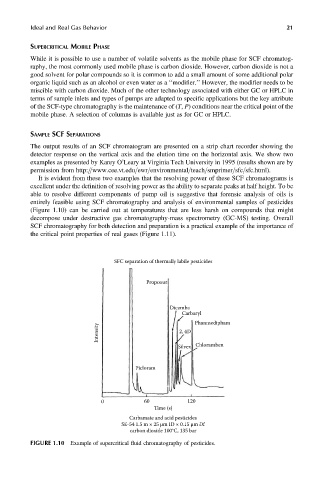
It is evident from these two examples that the resolving power of these SCF chromatograms is
excellent under the definition of resolving power as the ability to separate peaks at half height. To be
able to resolve different components of pump oil is suggestive that forensic analysis of oils is
entirely feasible using SCF chromatography and analysis of environmental samples of pesticides
(Figure 1.10) can be carried out at temperatures that are less harsh on compounds that might
decompose under destructive gas chromatography-mass spectrometry (GC-MS) testing. Overall
SCF chromatography for both detection and preparation is a practical example of the importance of
the critical point properties of real gases (Figure 1.11).
SFC separation of thermally labile pesticides
Propoxur
Dicamba
Carbaryl
Intensity 2, 4D Phanmedipham
Chloramben
Silvex
Picloram
120
0 60
Time (s)
Carbamate and acid pesticides
SE-54 1.5 m × 25 μm ID × 0.15 μm Df
carbon dioxide 100°C, 135 bar
FIGURE 1.10 Example of supercritical fluid chromatography of pesticides.