Page 84 - Essentials of physical chemistry
P. 84
46 Essentials of Physical Chemistry
d v
λ
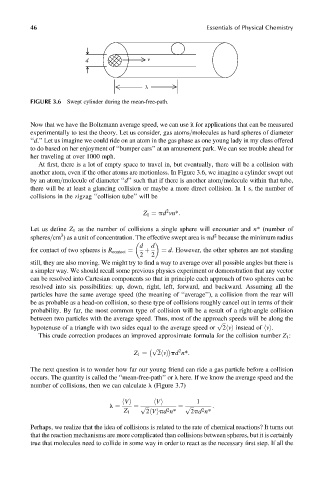
FIGURE 3.6 Swept cylinder during the mean-free-path.
Now that we have the Boltzmann average speed, we can use it for applications that can be measured
experimentally to test the theory. Let us consider, gas atoms=molecules as hard spheres of diameter
‘‘d.’’ Let us imagine we could ride on an atom in the gas phase as one young lady in my class offered
to do based on her enjoyment of ‘‘bumper cars’’ at an amusement park. We can see trouble ahead for
her traveling at over 1000 mph.
At first, there is a lot of empty space to travel in, but eventually, there will be a collision with
another atom, even if the other atoms are motionless. In Figure 3.6, we imagine a cylinder swept out
by an atom=molecule of diameter ‘‘d’’ such that if there is another atom=molecule within that tube,
there will be at least a glancing collision or maybe a more direct collision. In 1 s, the number of
collisions in the zigzag ‘‘collision tube’’ will be
2
Z 1 ¼ pd vn*:
Let us define Z 1 as the number of collisions a single sphere will encounter and n* (number of
2
3
spheres=cm ) as a unit of concentration. The effective swept area is pd because the minimum radius
d d
¼ d. However, the other spheres are not standing
for contact of two spheres is R contact ¼ þ
2 2
still, they are also moving. We might try to find a way to average over all possible angles but there is
a simpler way. We should recall some previous physics experiment or demonstration that any vector
can be resolved into Cartesian components so that in principle each approach of two spheres can be
resolved into six possibilities: up, down, right, left, forward, and backward. Assuming all the
particles have the same average speed (the meaning of ‘‘average’’), a collision from the rear will
be as probable as a head-on collision, so these type of collisions roughly cancel out in terms of their
probability. By far, the most common type of collision will be a result of a right-angle collision
between two particles with the average speed. Thus, most of the approach speeds will be along the
p ffiffiffi
hypotenuse of a triangle with two sides equal to the average speed or 2hvi instead of hvi.
This crude correction produces an improved approximate formula for the collision number Z 1 :
ffiffiffi 2
p
2hvi pd n*:
Z 1 ¼
The next question is to wonder how far our young friend can ride a gas particle before a collision
occurs. The quantity is called the ‘‘mean-free-path’’ or l here. If we know the average speed and the
number of collisions, then we can calculate l (Figure 3.7)
1
:
hVi hVi
ffiffiffi ffiffiffi
l ¼ ¼ p 2 ¼ p 2
Z 1 2hVipd n* 2pd n*
Perhaps, we realize that the idea of collisions is related to the rate of chemical reactions? It turns out
that the reaction mechanisms are more complicated than collisions between spheres, but it is certainly
true that molecules need to collide in some way in order to react as the necessary first step. If all the