Page 238 - Facility Piping Systems Handbook for Industrial, Commercial, and Healthcare Facilities
P. 238
HEAT TRANSFER, INSULATION, AND FREEZE PROTECTION
5.22 CHAPTER FIVE
THE MECHANICS OF THE FREEZING PROCESS
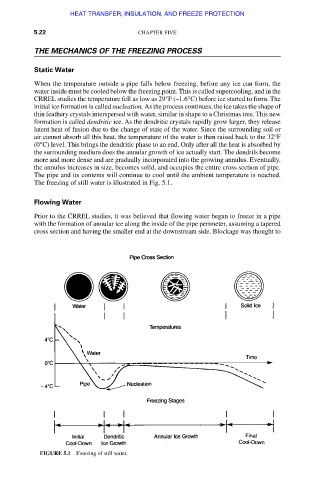
Static Water
When the temperature outside a pipe falls below freezing, before any ice can form, the
water inside must be cooled below the freezing point. This is called supercooling, and in the
CRREL studies the temperature fell as low as 29°F (–1.6°C) before ice started to form. The
initial ice formation is called nucleation. As the process continues, the ice takes the shape of
thin feathery crystals interspersed with water, similar in shape to a Christmas tree. This new
formation is called dendritic ice. As the dendritic crystals rapidly grow larger, they release
latent heat of fusion due to the change of state of the water. Since the surrounding soil or
air cannot absorb all this heat, the temperature of the water is then raised back to the 32°F
(0°C) level. This brings the dendritic phase to an end. Only after all the heat is absorbed by
the surrounding medium does the annular growth of ice actually start. The dendrils become
more and more dense and are gradually incorporated into the growing annulus. Eventually,
the annulus increases in size, becomes solid, and occupies the entire cross section of pipe.
The pipe and its contents will continue to cool until the ambient temperature is reached.
The freezing of still water is illustrated in Fig. 5.1.
Flowing Water
Prior to the CRREL studies, it was believed that flowing water began to freeze in a pipe
with the formation of annular ice along the inside of the pipe perimeter, assuming a tapered
cross section and having the smaller end at the downstream side. Blockage was thought to
FIGURE 5.1 Freezing of still water.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.accessengineeringlibrary.com)
Copyright © 2009 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.