Page 318 - Failure Analysis Case Studies II
P. 318
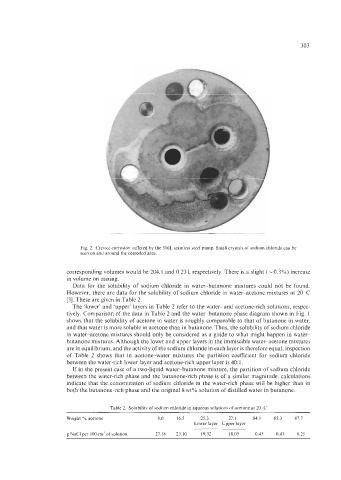
303
Fig. 2. Crevice corrosion suffered by the 316L stainless steel pump. Small crystals of sodium chloride can be
seen on and around the corroded area.
corresponding volumes would be 204.1 and 0.23 1, respectively. There is a slight (- 0.3%) increase
in volume on mixing.
Data for the solubility of sodium chloride in water-butanone mixtures could not be found.
However, there are data for the solubility of sodium chloride in water-acetone mixtures at 20 "C
[3]. These are given in Table 2.
The 'lower' and 'upper' layers in Table 2 refer to the water- and acetone-rich solutions, respec-
tively. Comparison of the data in Table 2 and the water-butanone phase diagram shown in Fig. 1
shows that the solubility of acetone in water is roughly comparable to that of butanone in water,
and that water is more soluble in acetone than in butanone. Thus, the solubility of sodium chloride
in water-acetone mixtures should only be considered as a guide to what might happen in water-
butanone mixtures. Although the lower and upper layers in the immiscible water-acetone mixtures
are in equilibrium, and the activity of the sodium chloride in each layer is therefore equal, inspection
of Table 2 shows that in acetone-water mixtures the partition coefficient for sodium chloride
between the water-rich lower layer and acetone-rich upper layer is 40: 1.
If in the present case of a two-liquid water-butanone mixture, the partition of sodium chloride
between the water-rich phase and the butanone-rich phase is of a similar magnitude, calculations
indicate that the concentration of sodium chloride in the water-rich phase will be higher than in
both the butanone-rich phase and the original 8 wt% solution of distilled water in butanone.
Table 2. Solubility of sodium chloride in aqueous solutions of acetone at 20 "C
Weight % acetone 8.0 16.5 25.3 27.1 84.1 85.3 87.7
Lower layer Upper layer
E NaCl Der 100 cm3 of solution 27.18 23.10 19.32 18.05 0.45 0.43 0.25