Page 315 - Failure Analysis Case Studies II
P. 315
300
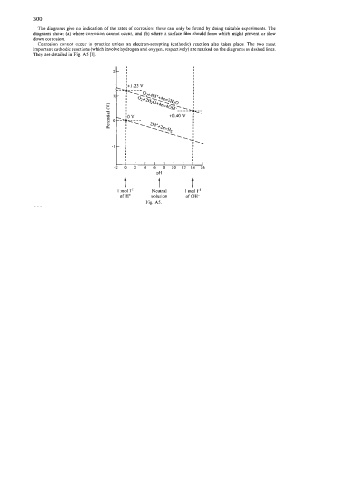
The diagrams give no indication of the rates of corrosion: these can only be found by doing suitable experiments. The
diagrams show: (a) where corrosion cannot occur, and (b) where a surface film should form which might prevent or slow
down corrosion.
Corrosion cannot occur in practice unless an electron-accepting (cathodic) reaction also takes place. The two most
important cathodic reactions (which involve hydrogen and oxygen, respectively) are marked on the diagrams as dashed lines.
They are detailed in Fig. A5 [I].
I
Ii I I
l ~ l l l l l l I i
-2 0 2 4 6 8 10 12 14 16
PH
I t
1 mol I-' Neutral I mol I-'
of H+ solution of OH-
Fig. A5.