Page 311 - Failure Analysis Case Studies II
P. 311
296
I l l I I! I I
-2 0 2 4 6 8 10 12 14 16
PH
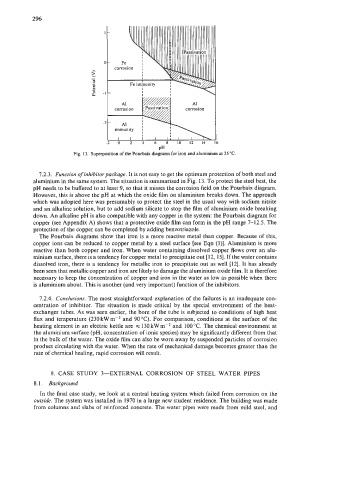
Fig. 13. Superposition of the Pourbaix diagrams for iron and aluminium at 25 "C.
7.2.3. Function of inhibitorpackage. It is not easy to get the optimum protection of both steel and
aluminium in the same system. The situation is summarized in Fig. 13. To protect the steel best, the
pH needs to be buffered to at least 9, so that it misses the corrosion field on the Pourbaix diagram.
However, this is above the pH at which the oxide film on aluminium breaks down. The approach
which was adopted here was presumably to protect the steel in the usual way with sodium nitrite
and an alkaline solution, but to add sodium silicate to stop the film of aluminium oxide breaking
down. An alkaline pH is also compatible with any copper in the system: the Pourbaix diagram for
copper (see Appendix A) shows that a protective oxide film can form in the pH range 7-12.5. The
protection of the copper can be completed by adding benzotriazole.
The Pourbaix diagrams show that iron is a more reactive metal than copper. Because of this,
copper ions can be reduced to copper metal by a steel surface [see Eqn (l)]. Aluminium is more
reactive than both copper and iron. When water containing dissolved copper flows over an alu-
minium surface, there is a tendency for copper metal to precipitate out [12,15]. If the water contains
dissolved iron, there is a tendency for metallic iron to precipitate out as well [12]. It has already
been seen that metallic copper and iron are likely to damage the aluminium oxide film. It is therefore
necessary to keep the concentration of copper and iron in the water as low as possible when there
is aluminium about. This is another (and very important) function of the inhibitors.
7.2.4. Conclusions. The most straightforward explanation of the failures is an inadequate con-
centration of inhibitor. The situation is made critical by the special environment of the heat-
exchanger tubes. As was seen earlier, the bore of the tube is subjected to conditions of high heat
flux and temperature (230 kW m-* and 90 "C). For comparison, conditions at the surface of the
heating element in an electric kettle are x 130 kW m-' and 100 "C. The chemical environment at
the aluminium surface (pH, concentration of ionic species) may be significantly different from that
in the bulk of the water. The oxide film can also be worn away by suspended particles of corrosion
product circulating with the water. When the rate of mechanical damage becomes greater than the
rate of chemical healing, rapid corrosion will result.
8. CASE STUDY 3-EXTERNAL CORROSION OF STEEL WATER PIPES
8.1. Background
In the final case study, we look at a central heating system which failed from corrosion on the
outside. The system was installed in 1970 in a large new student residence. The building was made
from columns and slabs of reinforced concrete. The water pipes were made from mild steel, and