Page 307 - Failure Analysis Case Studies II
P. 307
292
Concentration of NaN02
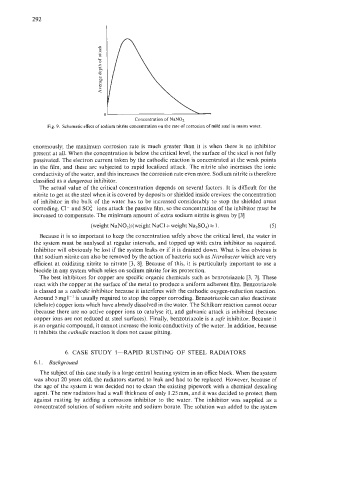
Fig. 9. Schematic effect of sodium nitrite concentration on the rate of corrosion of mild steel in mains water.
enormously; the maximum corrosion rate is much greater than it is when there is no inhibitor
present at all. When the concentration is below the critical level, the surface of the steel is not fully
passivated. The electron current taken by the cathodic reaction is concentrated at the weak points
in the film, and these are subjected to rapid localized attack. The nitrite also increases the ionic
conductivity of the water, and this increases the corrosion rate even more. Sodium nitrite is therefore
classified as a dangerous inhibitor.
The actual value of the critical concentration depends on several factors. It is difficult for the
nitrite to get at the steel when it is covered by deposits or shielded inside crevices: the concentration
of inhibitor in the bulk of the water has to be increased considerably to stop the shielded areas
corroding. C1- and SO:- ions attack the passive film, so the concentration of the inhibitor must be
increased to compensate. The minimum amount of extra sodium nitrite is given by [3]
(weight NaNO,)/(weight NaCl+ weight Na2SO4) % 1. (5)
Because it is so important to keep the concentration safely above the critical level, the water in
the system must be analysed at regular intervals, and topped up with extra inhibitor as required.
Inhibitor will obviously be lost if the system leaks or if it is drained down. What is less obvious is
that sodium nitrite can also be removed by the action of bacteria such as Nitrobacter which are very
efficient at oxidizing nitrite to nitrate [3, 81. Because of this, it is particularly important to use a
biocide in any system which relies on sodium nitrite for its protection.
The best inhibitors for copper are specific organic chemicals such as benzotriazole [3, 71. These
react with the copper at the surface of the metal to produce a uniform adherent film. Benzotriazole
is classed as a cathodic inhibitor because it interferes with the cathodic oxygen-reduction reaction.
Around 5 mg 1-' is usually required to stop the copper corroding. Benzotriazole can also deactivate
(chelate) copper ions which have already dissolved in the water. The Schikorr reaction cannot occur
(because there are no active copper ions to catalyse it), and galvanic attack is inhibited (because
copper ions are not reduced at steel surfaces). Finally, benzotriazole is a safe inhibitor. Because it
is an organic compound, it cannot increase the ionic conductivity of the water. In addition, because
it inhibits the cathodic reaction it does not cause pitting.
6. CASE STUDY 1-RAPID RUSTING OF STEEL RADIATORS
6.1. Background
The subject of this case study is a large central heating system in an office block. When the system
was about 20 years old, the radiators started to leak and had to be replaced. However, because of
the age of the system it was decided not to clean the existing pipework with a chemical descaling
agent. The new radiators had a wall thickness of only 1.25 mm, and it was decided to protect them
against rusting by adding a corrosion inhibitor to the water. The inhibitor was supplied as a
concentrated solution of sodium nitrite and sodium borate. The solution was addcd to the system