Page 308 - Failure Analysis Case Studies II
P. 308
293
I .25
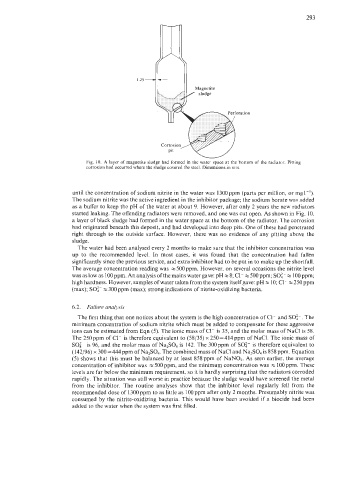
Fig. 10. A layer of magnetite sludge had formed in the water space at the bottom of the radiator. Pitting
corrosion had occurred where the sludge covered the steel. Dimensions in mm.
until the concentration of sodium nitrite in the water was 1300ppm (parts per million, or mgl-’).
The sodium nitrite was the active ingredient in the inhibitor package; the sodium borate was added
as a buffer to keep the pH of the water at about 9. However, after only 2 years the new radiators
started leaking. The offending radiators were removed, and one was cut open. As shown in Fig. 10,
a layer of black sludge had formed in the water space at the bottom of the radiator. The corrosion
had originated beneath this deposit, and had developed into deep pits. One of these had penetrated
right through to the outside surface. However, there was no evidence of any pitting above the
sludge.
The water had been analysed every 2 months to make sure that the inhibitor concentration was
up to the recommended level. In most cases, it was found that the Concentration had fallen
significantly since the previous service, and extra inhibitor had to be put in to make up the shortfall.
The average concentration reading was E 500 ppm. However, on several occasions the nitrite level
was as low as 100 ppm. An analysis of the mains water gave: pH E 8; C1- z 500 ppm; SO:- E 100 ppm;
high hardness. However, samples of water taken from the system itself gave: pH z 10; C1- z 250 ppm
(max); SO:- z 300 ppm (max); strong indications of nitrite-oxidizing bacteria.
6.2. Failure analysis
The first thing that one notices about the system is the high concentration of C1- and SO:-, The
minimum concentration of sodium nitrite which must be added to compensate for these aggressive
ions can be estimated from Eqn (5). The ionic mass of CI- is 35, and the molar mass of NaCl is 58.
The 250ppm of C1- is therefore equivalent to (58/35) x 250=414ppm of NaC1. The ionic mass of
SO:- is 96, and the molar mass of Na2S0, is 142. The 300ppm of SO:- is therefore equivalent to
(142/96) x 300 = 444 ppm of Na,SO,. The combined mass of NaCl and Na2S0, is 858 ppm. Equation
(5) shows that this must be balanced by at least 858ppm of NaNO,. As seen earlier, the average
concentration of inhibitor was E 500ppm, and the minimum concentration was E 100ppm. These
levels are far below the minimum requirement, so it is hardly surprising that the radiators corroded
rapidly. The situation was still worse in practice because the sludge would have screened the metal
from the inhibitor. The routine analyses show that the inhibitor level regularly fell from the
recommended dose of 1300ppm to as little as l0Oppm after only 2 months. Presumably nitrite was
consumed by the nitrite-oxidizing bacteria. This would have been avoided if a biocide had been
added to the water when the system was first filled.