Page 306 - Failure Analysis Case Studies II
P. 306
291
T ("0
80 75 70 65 - 60365
6
'I I
T" (K-')
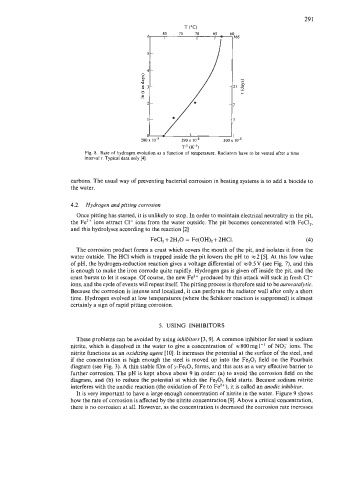
Fig. 8. Rate of hydrogen evolution as a function of temperature. Radiators have to be vented after a time
interval t. Typical data only [4].
carbons. The usual way of preventing bacterial corrosion in heating systems is to add a biocide to
the water.
4.2. Hydrogen andpitting corrosion
Once pitting has started, it is unlikely to stop. In order to maintain electrical neutrality in the pit,
the Fez+ ions attract C1- ions from the water outside. The pit becomes concentrated with FeCI,,
and this hydrolyses according to the reaction [2]
FeCl, + 2H20 = Fe(OH)* + 2HC1. (4)
The corrosion product forms a crust which covers the mouth of the pit, and isolates it from the
water outside. The HCl which is trapped inside the pit lowers the pH to x 2 [SI. At this low value
of pH, the hydrogen-reduction reaction gives a voltage differential of ~0.5 V (see Fig. 7), and this
is enough to make the iron corrode quite rapidly. Hydrogen gas is given off inside the pit, and the
crust bursts to let it escape. Of course, the new Fe2+ produced by this attack will suck in fresh C1-
ions, and the cycle of events will repeat itself. The pitting process is therefore said to be autocatalytic.
Because the corrosion is intense and localized, it can perforate the radiator wall after only a short
time. Hydrogen evolved at low temperatures (where the Schikorr reaction is suppressed) is almost
certainly a sign of rapid pitting corrosion.
5. USING INHIBITORS
These problems can be avoided by using inhibitors [3, 91. A common inhibitor for steel is sodium
nitrite, which is dissolved in the water to give a concentration of x800mg1-' of NO; ions. The
nitrite functions as an oxidizing agent [lo]. It increases the potential at the surface of the steel, and
if the concentration is high enough the steel is moved up into the Fe203 field on the Pourbaix
diagram (see Fig. 3). A thin stable film of y-Fe203 forms, and this acts as a very effective barrier to
further corrosion. The pH is kept above about 9 in order: (a) to avoid the corrosion field on the
diagram, and (b) to reduce the potential at which the Fe203 field starts. Because sodium nitrite
interferes with the anodic reaction (the oxidation of Fe to Fez+), it is called an anodic inhibitor.
It is very important to have a large enough concentration of nitrite in the water. Figure 9 shows
how the rate of corrosion is affected by the nitrite concentration [9]. Above a critical concentration,
there is no corrosion at all. However, as the concentration is decreased the corrosion rate increases