Page 301 - Failure Analysis Case Studies II
P. 301
286
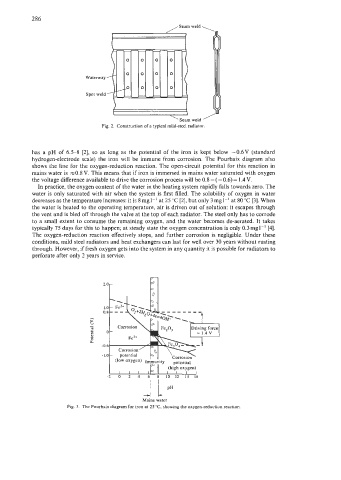
Waterway
Spot weld
Fig. 2. Construction of a typical mild-steel radiator.
has a pH of 6.5-8 [2], so as long as the potential of the iron is kept below -0.6V (standard
hydrogen-electrode scale) the iron will be immune from corrosion. The Pourbaix diagram also
shows the line for the oxygen-reduction reaction. The open-circuit potential for this reaction in
mains water is ~0.8 V. This means that if iron is immersed in mains water saturated with oxygen
the voltage difference available to drive the corrosion process will be 0.8 - (- 0.6) = 1.4 V.
In practice, the oxygen content of the water in the heating system rapidly falls towards zero. The
water is only saturated with air when the system is first filled. The solubility of oxygen in water
decreases as the temperature increases: it is 8 mg 1-' at 25 "C [2], but only 3 mg 1-' at 80 "C [3]. When
the water is heated to the operating temperature, air is driven out of solution: it escapes through
the vent and is bled off through the valve at the top of each radiator. The steel only has to corrode
to a small extent to consume the remaining oxygen, and the water becomes de-aerated. It takes
typically 75 days for this to happen; at steady state the oxygen concentration is only 0.3mgl-' [4].
The oxygen-reduction reaction effectively stops, and further corrosion is negligible. Under these
conditions, mild steel radiators and heat exchangers can last for well over 30 years without rusting
through. However, if fresh oxygen gets into the system in any quantity it is possible for radiators to
perforate after only 2 years in service.
2.0-
-1.0 - potential
I I I I I I I I
-2 0 2 4 6, 8Ll;H 12 14 16
+
Mains water
Fig. 3. The Pourbaix diagram for iron at 25 "C. showing the oxygen-reduction reaction.