Page 317 - Failure Analysis Case Studies II
P. 317
302
160 I I I 1
- -
140
- -
120
Y - -
@ 100
3
E Bo- -
n -
i 60-
I- -
2 liquids
f-
b L :,-100
Weight % Butanone
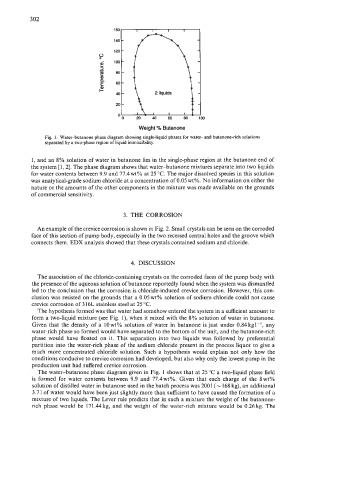
Fig. 1. Water-butanone phase diagram showing single-liquid phases for water- and butanone-rich solutions
separated by a two-phase region of liquid immiscibility.
1, and an 8% solution of water in butanone lies in the single-phase region at the butanone end of
the system [l, 21. The phase diagram shows that water-butanone mixtures separate into two liquids
for water contents between 9.9 and 77.4wtY0 at 25 "C. The major dissolved species in this solution
was analytical-grade sodium chloride at a concentration of 0.05 wt%. No information on either the
nature or the amounts of the other components in the mixture was made available on the grounds
of commercial sensitivity.
3. THE CORROSION
An example of the crevice corrosion is shown in Fig. 2. Small crystals can be seen on the corroded
face of this section of pump body, especially in the two recessed central holes and the groove which
connects them. EDX analysis showed that these crystals contained sodium and chloride.
4. DISCUSSION
The association of the chloride-containing crystals on the corroded faces of the pump body with
the presence of the aqueous solution of butanone reportedly found when the system was dismantled
led to the conclusion that the corrosion is chloride-induced crevice corrosion. However, this con-
clusion was resisted on the grounds that a 0.05 wt% solution of sodium chloride could not cause
crevice corrosion of 316L stainless steel at 25 "C.
The hypothesis formed was that water had somehow entered the system in a sufficient amount to
form a two-liquid mixture (see Fig. l), when it mixed with the 8% solution of water in butanone.
Given that the density of a IOwt% solution of water in butanone is just under 0.84kg1-', any
water-rich phase so formed would have separated to the bottom of the unit, and the butanone-rich
phase would have floated on it. This separation into two liquids was followed by preferential
partition into the water-rich phase of the sodium chloride present in the process liquor to give a
much more concentrated chloride solution. Such a hypothesis would explain not only how the
conditions conducive to crevice corrosion had developed, but also why only the lowest pump in the
production unit had suffered crevice corrosion.
The water-butanone phase diagram given in Fig. 1 shows that at 25 "C a two-liquid phase field
is formed for water contents between 9.9 and 77.4wt%. Given that each charge of the 8wt%
solution of distilled water in butanone used in the batch process was 200 1 ( N 168 kg), an additional
3.71 of water would have been just slightly more than sufficient to have caused the formation of a
mixture of two liquids. The Lever rule predicts that in such a mixture the weight of the butanone-
rich phase would be 171.44kg, and the weight of the water-rich mixture would be 0.26kg. The