Page 237 - Fundamentals of Air Pollution 3E
P. 237
200 14. Ambient Air Pollutant Analysis and Measurement
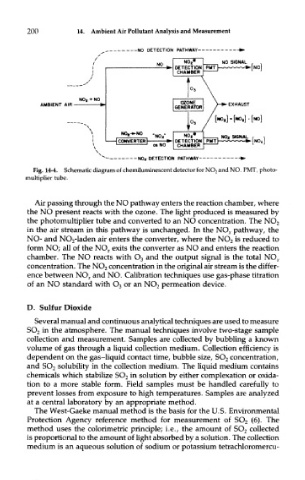
Fig. 14-4. Schematic diagram of chemiluminescent detector for NO 2 and NO. PMT, photo-
multiplier tube.
Ak passing through the NO pathway enters the reaction chamber, where
the NO present reacts with the ozone. The light produced is measured by
the photomultiplier tube and converted to an NO concentration. The NO 2
in the air stream in this pathway is unchanged. In the NO X pathway, the
NO- and NO 2-laden air enters the converter, where the NO 2 is reduced to
form NO; all of the NO.,, exits the converter as NO and enters the reaction
chamber. The NO reacts with O 3 and the output signal is the total NQ X
concentration. The NO 2 concentration in the original air stream is the differ-
ence between NO,, and NO. Calibration techniques use gas-phase titration
of an NO standard with O 3 or an NO 2 permeation device.
D, Sulfur Dioxide
Several manual and continuous analytical techniques are used to measure
SO 2 in the atmosphere. The manual techniques involve two-stage sample
collection and measurement. Samples are collected by bubbling a known
volume of gas through a liquid collection medium. Collection efficiency is
dependent on the gas-liquid contact time, bubble size, SO 2 concentration,
and SO 2 solubility in the collection medium. The liquid medium contains
chemicals which stabilize SO 2 in solution by either complexation or oxida-
tion to a more stable form. Field samples must be handled carefully to
prevent losses from exposure to high temperatures. Samples are analyzed
at a central laboratory by an appropriate method.
The West-Gaeke manual method is the basis for the U.S. Environmental
Protection Agency reference method for measurement of SO 2 (6). The
method uses the colorimetric principle; i.e., the amount of SO 2 collected
is proportional to the amount of light absorbed by a solution. The collection
medium is an aqueous solution of sodium or potassium tetrachloromercu-