Page 162 - Fundamentals of Air Pollution
P. 162
II. Effects on Stone 129
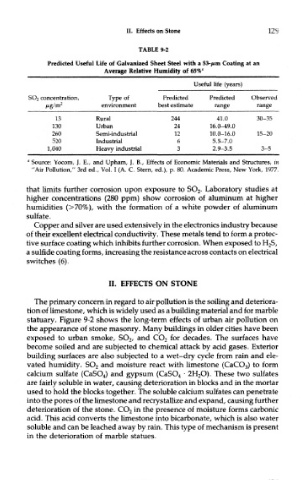
TABLE 9-2
Predicted Useful Life of Galvanized Sheet Steel with a 53-/nm Coating at an
Average Relative Humidity of 65% fl
Useful life (years)
SO 2 concentration, Type of Predicted Predicted Observed
jWg/m 2 environment best estimate range range
13 Rural 244 41.0 30-35
130 Urban 24 16.0-49.0
260 Semi-industrial 12 10.0-16.0 15-20
520 Industrial 6 5.5-7.0
1,040 Heavy industrial 3 2.9-3.5 3-5
" Source: Yocom, J. E., and Upham, J. B., Effects of Economic Materials and Structures, in
"Air Pollution," 3rd ed., Vol. I (A. C. Stern, ed.), p. 80. Academic Press, New York, 1977.
that limits further corrosion upon exposure to SO 2. Laboratory studies at
higher concentrations (280 ppm) show corrosion of aluminum at higher
humidities (>70%), with the formation of a white powder of aluminum
sulfate.
Copper and silver are used extensively in the electronics industry because
of their excellent electrical conductivity. These metals tend to form a protec-
tive surface coating which inhibits further corrosion. When exposed to H 2S,
a sulfide coating forms, increasing the resistance across contacts on electrical
switches (6).
II. EFFECTS ON STONE
The primary concern in regard to air pollution is the soiling and deteriora-
tion of limestone, which is widely used as a building material and for marble
statuary. Figure 9-2 shows the long-term effects of urban air pollution on
the appearance of stone masonry. Many buildings in older cities have been
exposed to urban smoke, SO 2/ and CO 2 for decades. The surfaces have
become soiled and are subjected to chemical attack by acid gases. Exterior
building surfaces are also subjected to a wet-dry cycle from rain and ele-
vated humidity. SO 2 and moisture react with limestone (CaCO 3) to form
calcium sulfate (CaSO 4) and gypsum (CaSO 4 • 2H 2O). These two sulfates
are fairly soluble in water, causing deterioration in blocks and in the mortar
used to hold the blocks together. The soluble calcium sulfates can penetrate
into the pores of the limestone and recrystallize and expand, causing further
deterioration of the stone. CO 2 in the presence of moisture forms carbonic
acid. This acid converts the limestone into bicarbonate, which is also water
soluble and can be leached away by rain. This type of mechanism is present
in the deterioration of marble statues.