Page 159 - Fundamentals of Air Pollution
P. 159
I. Effects on Metals 127
ates the corrosion of metals. Metal surfaces can by wetted repeatedly over
a period of time as the humidity fluctuates.
Several studies have been conducted in urban areas to relate air pollution
exposure and metal corrosion. In Tulsa, Oklahoma, wrought iron disks
were exposed in various locations (2). Using weight change as a measure
of air pollution corrosion, the results indicated higher corrosion rates near
industrial sectors containing an oil refinery and fertilizer and sulfuric acid
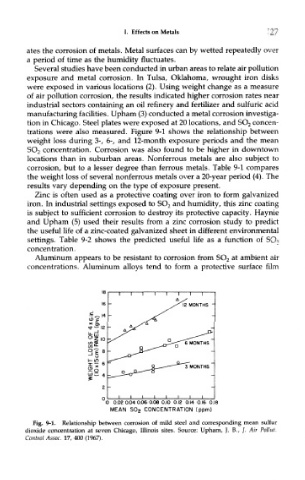
manufacturing facilities. Upham (3) conducted a metal corrosion investiga-
tion in Chicago. Steel plates were exposed at 20 locations, and SO 2 concen-
trations were also measured. Figure 9-1 shows the relationship between
weight loss during 3-, 6-, and 12-month exposure periods and the mean
SO 2 concentration. Corrosion was also found to be higher in downtown
locations than in suburban areas. Nonferrous metals are also subject to
corrosion, but to a lesser degree than ferrous metals. Table 9-1 compares
the weight loss of several nonferrous metals over a 20-year period (4). The
results vary depending on the type of exposure present.
Zinc is often used as a protective coating over iron to form galvanized
iron. In industrial settings exposed to SO 2 and humidity, this zinc coating
is subject to sufficient corrosion to destroy its protective capacity. Haynie
and Upham (5) used their results from a zinc corrosion study to predict
the useful life of a zinc-coated galvanized sheet in different environmental
settings. Table 9-2 shows the predicted useful life as a function of SO 2
concentration.
Aluminum appears to be resistant to corrosion from SO 2 at ambient air
concentrations. Aluminum alloys tend to form a protective surface film
Fig. 9-1. Relationship between corrosion of mild steel and corresponding mean sulfur
dioxide concentration at seven Chicago, Illinois sites. Source: Upham, J. B., /. Air Pollut.
Control Assoc. 17, 400 (1967).