Page 201 - Fundamentals of Enhanced Oil and Gas Recovery
P. 201
Chemical Flooding 189
1.20
Wagner and Leach
1.00
0.80 Foster
Sor/Sor, wf 0.60 Moore and Slobod Du prey
Abrams
0.40 Taber Du prey
0.20
0.00
1.0E – 08 1.0E – 06 1.0E – 04 1.0E – 02 1.0E + 00
Capillary number, Nc
Nonwetting residual
Wetting residual
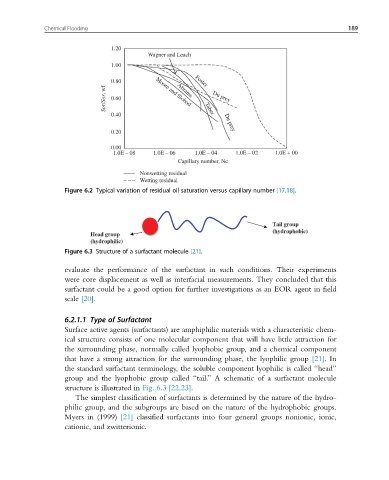
Figure 6.2 Typical variation of residual oil saturation versus capillary number [17,18].
Tail group
(hydrophobic)
Head group
(hydrophilic)
Figure 6.3 Structure of a surfactant molecule [21].
evaluate the performance of the surfactant in such conditions. Their experiments
were core displacement as well as interfacial measurements. They concluded that this
surfactant could be a good option for further investigations as an EOR agent in field
scale [20].
6.2.1.1 Type of Surfactant
Surface active agents (surfactants) are amphiphilic materials with a characteristic chem-
ical structure consists of one molecular component that will have little attraction for
the surrounding phase, normally called lyophobic group, and a chemical component
that have a strong attraction for the surrounding phase, the lyophilic group [21].In
the standard surfactant terminology, the soluble component lyophilic is called “head”
group and the lyophobic group called “tail.” A schematic of a surfactant molecule
structure is illustrated in Fig. 6.3 [22,23].
The simplest classification of surfactants is determined by the nature of the hydro-
philic group, and the subgroups are based on the nature of the hydrophobic groups.
Myers in (1999) [21] classified surfactants into four general groups nonionic, ionic,
cationic, and zwitterionic.