Page 203 - Fundamentals of Enhanced Oil and Gas Recovery
P. 203
Chemical Flooding 191
the worldwide surfactant production [25]. A simple example of this type of material is
3-dimethyldodecylamine propanesulphonate; within this group, there are a number of
important naturally occurring materials, known as triglycerides, a good example being
lecithin, which occurs in the membranes of many animal cells [22 24].
6.2.1.2 Concerns Associated With Surfactant Flooding
One of the main concerns associated with surfactant flooding is surfactant adsorption
from injected fluid onto the reservoir rock. This means that the concentration of sur-
factant decreases during the injection process, and this issue results in reduction of
performance of surfactant in oil recovery. Because the ability of surfactant in IFT
reduction or wettability alteration of the rock surface highly depends on the surfactant
content of the injected fluid.
Ahmadi and Shadizadeh [26] examined adsorption of a plant-based surfactant on to
real carbonate reservoir sample. In their experiments, increasing surfactant content of
the solution until a certain point resulted in increasing the amount of adsorption.
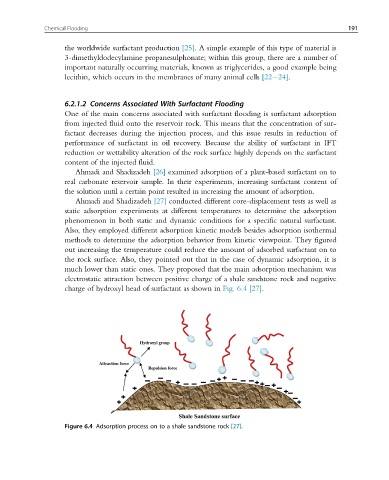
Ahmadi and Shadizadeh [27] conducted different core-displacement tests as well as
static adsorption experiments at different temperatures to determine the adsorption
phenomenon in both static and dynamic conditions for a specific natural surfactant.
Also, they employed different adsorption kinetic models besides adsorption isothermal
methods to determine the adsorption behavior from kinetic viewpoint. They figured
out increasing the temperature could reduce the amount of adsorbed surfactant on to
the rock surface. Also, they pointed out that in the case of dynamic adsorption, it is
much lower than static ones. They proposed that the main adsorption mechanism was
electrostatic attraction between positive charge of a shale sandstone rock and negative
charge of hydroxyl head of surfactant as shown in Fig. 6.4 [27].
Figure 6.4 Adsorption process on to a shale sandstone rock [27].