Page 199 - Fundamentals of Light Microscopy and Electronic Imaging
P. 199
182 FLUORESCENCE MICROSCOPY
Stokes shift
A E
Absorbance Emission
350 400 450 500 550 600 650
Wavelength (nm)
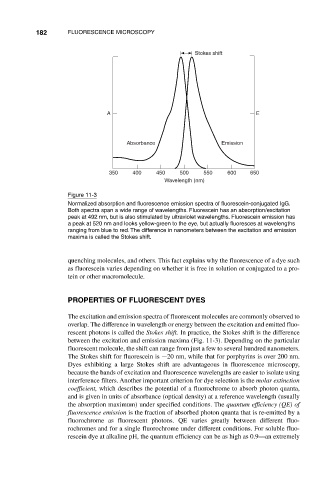
Figure 11-3
Normalized absorption and fluorescence emission spectra of fluorescein-conjugated IgG.
Both spectra span a wide range of wavelengths. Fluorescein has an absorption/excitation
peak at 492 nm, but is also stimulated by ultraviolet wavelengths. Fluorescein emission has
a peak at 520 nm and looks yellow-green to the eye, but actually fluoresces at wavelengths
ranging from blue to red. The difference in nanometers between the excitation and emission
maxima is called the Stokes shift.
quenching molecules, and others. This fact explains why the fluorescence of a dye such
as fluorescein varies depending on whether it is free in solution or conjugated to a pro-
tein or other macromolecule.
PROPERTIES OF FLUORESCENT DYES
The excitation and emission spectra of fluorescent molecules are commonly observed to
overlap. The difference in wavelength or energy between the excitation and emitted fluo-
rescent photons is called the Stokes shift. In practice, the Stokes shift is the difference
between the excitation and emission maxima (Fig. 11-3). Depending on the particular
fluorescent molecule, the shift can range from just a few to several hundred nanometers.
The Stokes shift for fluorescein is 20 nm, while that for porphyrins is over 200 nm.
Dyes exhibiting a large Stokes shift are advantageous in fluorescence microscopy,
because the bands of excitation and fluorescence wavelengths are easier to isolate using
interference filters. Another important criterion for dye selection is the molar extinction
coefficient, which describes the potential of a fluorochrome to absorb photon quanta,
and is given in units of absorbance (optical density) at a reference wavelength (usually
the absorption maximum) under specified conditions. The quantum efficiency (QE) of
fluorescence emission is the fraction of absorbed photon quanta that is re-emitted by a
fluorochrome as fluorescent photons. QE varies greatly between different fluo-
rochromes and for a single fluorochrome under different conditions. For soluble fluo-
rescein dye at alkaline pH, the quantum efficiency can be as high as 0.9—an extremely