Page 89 - Fundamentals of Physical Volcanology
P. 89
9780632054435_4_005.qxd 12/10/2007 12:20PM Page 66
66 CHAPTER 5
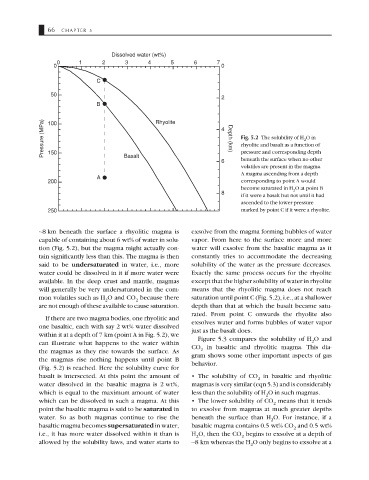
Dissolved water (wt%)
0 1 2 3 4 5 6 7
0 0
C
50
2
B
Pressure (MPa) 100 Rhyolite 4 Depth (km) rhyolite and basalt as a function of
Fig. 5.2 The solubility of H O in
2
150
Basalt
beneath the surface when no other
6 pressure and corresponding depth
volatiles are present in the magma.
A magma ascending from a depth
A
200 corresponding to point A would
become saturated in H O at point B
8 2
if it were a basalt but not until it had
ascended to the lower pressure
250 marked by point C if it were a rhyolite.
∼8 km beneath the surface a rhyolitic magma is exsolve from the magma forming bubbles of water
capable of containing about 6 wt% of water in solu- vapor. From here to the surface more and more
tion (Fig. 5.2), but the magma might actually con- water will exsolve from the basaltic magma as it
tain significantly less than this. The magma is then constantly tries to accommodate the decreasing
said to be undersaturated in water, i.e., more solubility of the water as the pressure decreases.
water could be dissolved in it if more water were Exactly the same process occurs for the rhyolite
available. In the deep crust and mantle, magmas except that the higher solubility of water in rhyolite
will generally be very undersaturated in the com- means that the rhyolitic magma does not reach
mon volatiles such as H O and CO because there saturation until point C (Fig. 5.2), i.e., at a shallower
2 2
are not enough of these available to cause saturation. depth than that at which the basalt became satu-
rated. From point C onwards the rhyolite also
If there are two magma bodies, one rhyolitic and
exsolves water and forms bubbles of water vapor
one basaltic, each with say 2 wt% water dissolved
just as the basalt does.
within it at a depth of 7 km (point A in Fig. 5.2), we
Figure 5.3 compares the solubility of H O and
can illustrate what happens to the water within 2
CO in basaltic and rhyolitic magma. This dia-
the magmas as they rise towards the surface. As 2
gram shows some other important aspects of gas
the magmas rise nothing happens until point B
behavior.
(Fig. 5.2) is reached. Here the solubility curve for
basalt is intersected. At this point the amount of • The solubility of CO in basaltic and rhyolitic
2
water dissolved in the basaltic magma is 2 wt%, magmas is very similar (eqn 5.3) and is considerably
which is equal to the maximum amount of water less than the solubility of H O in such magmas.
2
which can be dissolved in such a magma. At this • The lower solubility of CO means that it tends
2
point the basaltic magma is said to be saturated in to exsolve from magmas at much greater depths
water. So as both magmas continue to rise the beneath the surface than H O. For instance, if a
2
basaltic magma becomes supersaturated in water, basaltic magma contains 0.5 wt% CO and 0.5 wt%
2
i.e., it has more water dissolved within it than is H O, then the CO begins to exsolve at a depth of
2 2
allowed by the solubility laws, and water starts to ∼8 km whereas the H O only begins to exsolve at a
2