Page 90 - Fundamentals of Physical Volcanology
P. 90
9780632054435_4_005.qxd 12/10/2007 12:20PM Page 67
THE ROLE OF VOLATILES 67
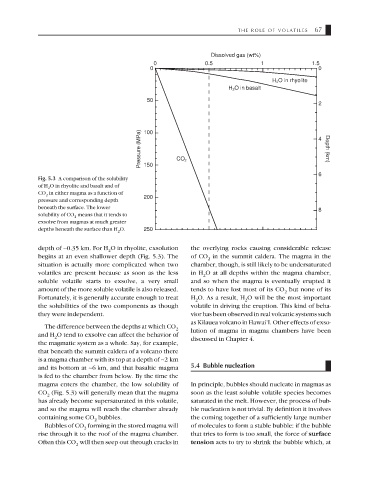
Dissolved gas (wt%)
0 0.5 1 1.5
0 0
H 2 O in rhyolite
H 2 O in basalt
50
2
Pressure (MPa) 100 CO 2 4 Depth (km)
150
6
Fig. 5.3 A comparison of the solubility
of H O in rhyolite and basalt and of
2
CO in either magma as a function of
2 200
pressure and corresponding depth
beneath the surface. The lower
8
solubility of CO means that it tends to
2
exsolve from magmas at much greater
depths beneath the surface than H O. 250
2
depth of ∼0.35 km. For H O in rhyolite, exsolution the overlying rocks causing considerable release
2
begins at an even shallower depth (Fig. 5.3). The of CO in the summit caldera. The magma in the
2
situation is actually more complicated when two chamber, though, is still likely to be undersaturated
volatiles are present because as soon as the less in H O at all depths within the magma chamber,
2
soluble volatile starts to exsolve, a very small and so when the magma is eventually erupted it
amount of the more soluble volatile is also released. tends to have lost most of its CO but none of its
2
Fortunately, it is generally accurate enough to treat H O. As a result, H O will be the most important
2
2
the solubilities of the two components as though volatile in driving the eruption. This kind of beha-
they were independent. vior has been observed in real volcanic systems such
as Kilauea volcano in Hawai’I. Other effects of exso-
The difference between the depths at which CO
2 lution of magma in magma chambers have been
and H O tend to exsolve can affect the behavior of
2 discussed in Chapter 4.
the magmatic system as a whole. Say, for example,
that beneath the summit caldera of a volcano there
is a magma chamber with its top at a depth of ∼2km
5.4 Bubble nucleation
and its bottom at ∼6 km, and that basaltic magma
is fed to the chamber from below. By the time the
magma enters the chamber, the low solubility of In principle, bubbles should nucleate in magmas as
CO (Fig. 5.3) will generally mean that the magma soon as the least soluble volatile species becomes
2
has already become supersaturated in this volatile, saturated in the melt. However, the process of bub-
and so the magma will reach the chamber already ble nucleation is not trivial. By definition it involves
containing some CO bubbles. the coming together of a sufficiently large number
2
Bubbles of CO forming in the stored magma will of molecules to form a stable bubble: if the bubble
2
rise through it to the roof of the magma chamber. that tries to form is too small, the force of surface
Often this CO will then seep out through cracks in tension acts to try to shrink the bubble which, at
2