Page 619 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 619
574 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
4
7 6
5
3
8
1
2
9
(a) (b)
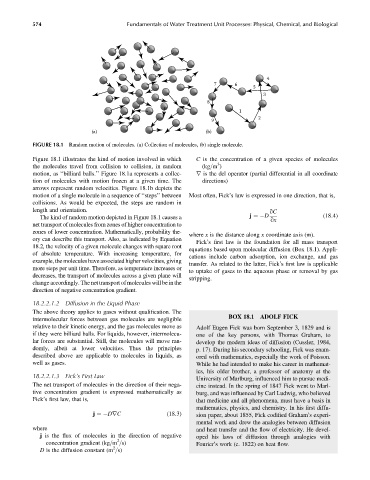
FIGURE 18.1 Random motion of molecules. (a) Collection of molecules, (b) single molecule.
Figure 18.1 illustrates the kind of motion involved in which C is the concentration of a given species of molecules
3
the molecules travel from collision to collision, in random (kg=m )
motion, as ‘‘billiard balls.’’ Figure 18.1a represents a collec- r is the del operator (partial differential in all coordinate
tion of molecules with motion frozen at a given time. The directions)
arrows represent random velocities. Figure 18.1b depicts the
motion of a single molecule in a sequence of ‘‘steps’’ between Most often, Fick’s law is expressed in one direction, that is,
collisions. As would be expected, the steps are random in
length and orientation. qC
The kindofrandommotiondepictedinFigure18.1causesa j ¼ D (18:4)
qx
net transport of molecules from zones of higher concentration to
zones of lower concentration. Mathematically, probability the-
where x is the distance along x coordinate axis (m).
ory can describe this transport. Also, as indicated by Equation
Fick’s first law is the foundation for all mass transport
18.2, the velocity of a given molecule changes with square root
equations based upon molecular diffusion (Box 18.1). Appli-
of absolute temperature. With increasing temperature, for
cations include carbon adsorption, ion exchange, and gas
example, the molecules have associated higher velocities, giving
transfer. As related to the latter, Fick’s first law is applicable
more steps per unit time. Therefore, as temperature increases or
to uptake of gases to the aqueous phase or removal by gas
decreases, the transport of molecules across a given plane will
stripping.
change accordingly. The net transport of molecules will be in the
direction of negative concentration gradient.
18.2.2.1.2 Diffusion in the Liquid Phase
The above theory applies to gases without qualification. The
BOX 18.1 ADOLF FICK
intermolecular forces between gas molecules are negligible
relative to their kinetic energy, and the gas molecules move as Adolf Eugen Fick was born September 3, 1829 and is
if they were billiard balls. For liquids, however, intermolecu- one of the key persons, with Thomas Graham, to
lar forces are substantial. Still, the molecules will move ran- develop the modern ideas of diffusion (Cussler, 1984,
domly, albeit at lower velocities. Thus the principles p. 17). During his secondary schooling, Fick was enam-
described above are applicable to molecules in liquids, as ored with mathematics, especially the work of Poisson.
well as gases. While he had intended to make his career in mathemat-
ics, his older brother, a professor of anatomy at the
18.2.2.1.3 Fick’s First Law
University of Marlburg, influenced him to pursue medi-
The net transport of molecules in the direction of their nega- cine instead. In the spring of 1847 Fick went to Marl-
tive concentration gradient is expressed mathematically as burg, and was influenced by Carl Ludwig, who believed
Fick’s first law, that is, that medicine and all phenomena, must have a basis in
mathematics, physics, and chemistry. In his first diffu-
j ¼ DrC (18:3) sion paper, about 1855, Fick codified Graham’s experi-
mental work and drew the analogies between diffusion
where and heat transfer and the flow of electricity. He devel-
j is the flux of molecules in the direction of negative oped his laws of diffusion through analogies with
2
concentration gradient (kg=m =s) Fourier’s work (c. 1822) on heat flow.
2
D is the diffusion constant (m =s)