Page 692 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 692
Oxidation 647
The relationship between DG and E is DG ¼ zFE, where The high negative free energy means that the reaction is
z ¼ equivalents per mole and F ¼ coulombs per equivalent. spontaneous. However, the reaction may be less strong than
o
For reference, in 1887, Walther Nernst started as the prin- what appears from the DG value, since the reaction rate, that
cipal assistant to Wilhelm Ostwald at Leipzig, one of the three is, kinetics, may vary over eight orders of magnitude, depend-
founders of physical chemistry, along with Svante Arrhenius ing on the substance reacting.
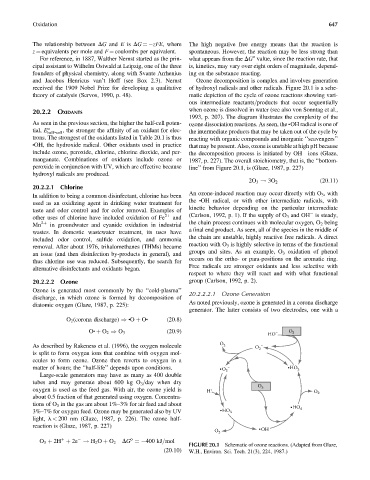
and Jacobus Henricus van’t Hoff (see Box 2.3). Nernst Ozone decomposition is complex and involves generation
received the 1909 Nobel Prize for developing a qualitative of hydroxyl radicals and other radicals. Figure 20.1 is a sche-
theory of catalysis (Servos, 1990, p. 48). matic depiction of the cycle of ozone reactions showing vari-
ous intermediate reactants=products that occur sequentially
when ozone is dissolved in water (see also von Sonntag et al.,
20.2.2 OXIDANTS
1993, p. 207). The diagram illustrates the complexity of the
As seen in the previous section, the higher the half-cell poten- ozone dissociation reactions. As seen, the . OH radical is one of
tial, E o , the stronger the affinity of an oxidant for elec-
half-cell the intermediate products that may be taken out of the cycle by
trons. The strongest of the oxidants listed in Table 20.1 is thus reacting with organic compounds and inorganic ‘‘scavengers’’
. OH, the hydroxide radical. Other oxidants used in practice
that may be present. Also, ozone is unstable at high pH because
include ozone, peroxide, chlorine, chlorine dioxide, and per- the decomposition process is initiated by OH ions (Glaze,
manganate. Combinations of oxidants include ozone or 1987, p. 227). The overall stoichiometry, that is, the ‘‘bottom-
peroxide in conjunction with UV, which are effective because line’’ from Figure 20.1, is (Glaze, 1987, p. 227)
hydroxyl radicals are produced.
(20:11)
2O 3 ! 3O 2
20.2.2.1 Chlorine
An ozone-induced reaction may occur directly with O 3 , with
In addition to being a common disinfectant, chlorine has been
the OH radical, or with other intermediate radicals, with
.
used as an oxidizing agent in drinking water treatment for
kinetic behavior depending on the particular intermediate
taste and odor control and for color removal. Examples of
(Carlson, 1992, p. 1). If the supply of O 3 and OH is steady,
other uses of chlorine have included oxidation of Fe 2þ and
the chain process continues with molecular oxygen, O 2 being
Mn 2þ in groundwater and cyanide oxidation in industrial
a final end product. As seen, all of the species in the middle of
wastes. In domestic wastewater treatment, its uses have
the chain are unstable, highly reactive free radicals. A direct
included odor control, sulfide oxidation, and ammonia
reaction with O 3 is highly selective in terms of the functional
removal. After about 1976, trihalomethanes (THMs) became
groups and sites. As an example, O 3 oxidation of phenol
an issue (and then disinfection by-products in general), and
occurs on the ortho- or para-positions on the aromatic ring.
thus chlorine use was reduced. Subsequently, the search for
Free radicals are stronger oxidants and less selective with
alternative disinfectants and oxidants began.
respect to where they will react and with what functional
20.2.2.2 Ozone group (Carlson, 1992, p. 2).
Ozone is generated most commonly by the ‘‘cold-plasma’’
20.2.2.2.1 Ozone Generation
discharge, in which ozone is formed by decomposition of
As noted previously, ozone is generated in a corona discharge
diatomic oxygen (Glaze, 1987, p. 225):
generator. The latter consists of two electrodes, one with a
O 2 (corona discharge) ) . O þ O . (20:8)
O . þ O 2 ) O 3 (20:9) – O 3
HO
O
As described by Rakeness et al. (1996), the oxygen molecule 2 O 2 –
is split to form oxygen ions that combine with oxygen mol-
ecules to form ozone. Ozone then reverts to oxygen in a
matter of hours; the ‘‘half-life’’ depends upon conditions. – HO 2
O 3
Large-scale generators may have as many as 400 double
tubes and may generate about 600 kg O 3 =day when dry
O 3
oxygen is used as the feed gas. With air, the ozone yield is H + O 2
about 0.5 fraction of that generated using oxygen. Concentra-
tions of O 3 in the gas are about 1%–3% for air feed and about
HO 4
3%–7% for oxygen feed. Ozone may be generated also by UV HO 3
light, l < 200 nm (Glaze, 1987, p. 226). The ozone half-
reaction is (Glaze, 1987, p. 227)
O 2 OH
o
O 3 þ 2H þ 2e ! H 2 O þ O 2 DG ¼ 400 kJ=mol
þ
FIGURE 20.1 Schematic of ozone reactions. (Adapted from Glaze,
(20:10) W.H., Environ. Sci. Tech. 21(3), 224, 1987.)