Page 696 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 696
Oxidation 651
100
2+
200 μg/L C(Mn , raw)= 200 μg/L
100 C(CIO 2 )=0.85 μg/L
2+
C(Mn , t=0)=60 μg/L TOC= 3.4 mg/L Dissolved Dissolved Colloidal
pH= 7.0 pH =7.4, T =5°C
80
T =9°C
80 Particulate
Permanganate—0.36 mg/L 60
C(Mn 2+ ) ( μg/L) 60 C(Mn 2+ ) (μm/L) 40 Particulate
40
Colloidal Dissolved Dissolved Colloidal Particulate Dissolved Colloidal Particulate
20 Ozone—1.5 mg/L 20 Particulate Dissolved
Chlorine dioxide—0.5 mg/L Colloidal
0 0
0 100 200 300 400 500 600 Raw Pre Post Settled Filtered
- rapid -rapid
Time (s)
-mix -mix
(a) (b) Stage in treatment
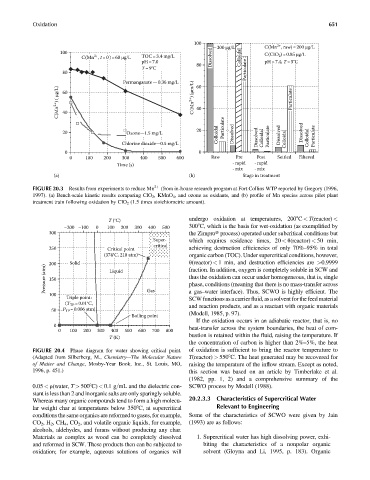
FIGURE 20.3 Results from experiments to reduce Mn 2þ (from in-house research program at Fort Collins WTP reported by Gregory (1996,
1997). (a) Bench-scale kinetic results comparing ClO 2 , KMnO 4 , and ozone as oxidants, and (b) profile of Mn species across pilot plant
treatment train following oxidation by ClO 2 (1.5 times stoichiometric amount).
undergo oxidation at temperatures, 2008C < T(reactor) <
T (°C)
–200 –100 0 100 200 300 400 500 3008C, which is the basis for wet-oxidation (as exemplified by
300 the Zimprot process) operated under subcritical conditions but
Super- which requires residence times, 20 < u(reactor) < 50 min,
critical
250 Critical point achieving destruction efficiencies of only 70%–95% in total
(374°C, 218 atm) organic carbon (TOC). Under supercritical conditions, however,
200 Solid Liquid u(reactor) < 1 min, and destruction efficiencies are >0.9999
Pressure (atm) 150 thus the oxidation can occur under homogeneous, that is, single
fraction. In addition, oxygen is completely soluble in SCW and
phase, conditions (meaning that there is no mass-transfer across
100 Gas agas–water interface). Thus, SCWO is highly efficient. The
Triple point: SCWfunctions as a carrier fluid,asa solvent for the feedmaterial
(T TP =0.01°C, and reaction products, and as a reactant with organic materials
50 P TP = 0.006 atm)
Boiling point (Modell, 1985, p. 97).
If the oxidation occurs in an adiabatic reactor, that is, no
0
0 100 200 300 400 500 600 700 800 heat-transfer across the system boundaries, the heat of com-
bustion is retained within the fluid, raising the temperature. If
T (K)
the concentration of carbon is higher than 2%–5%, the heat
FIGURE 20.4 Phase diagram for water showing critical point. of oxidation is sufficient to bring the reactor temperature to
(Adapted from Silberberg, M., Chemistry—The Molecular Nature T(reactor) > 5508C. The heat generated may be recovered for
of Matter and Change, Mosby-Year Book, Inc., St. Louis, MO, raising the temperature of the inflow stream. Except as noted,
1996, p. 451.) this section was based on an article by Timberlake et al.
(1982, pp. 1, 2) and a comprehensive summary of the
0.05< r(water, T > 5008C) < 0.1 g=mL and the dielectric con- SCWO process by Modell (1988).
stant is less than 2 and inorganic salts are only sparingly soluble.
20.2.3.3 Characteristics of Supercritical Water
Whereas many organic compounds tend to form a high molecu-
lar weight char at temperatures below 3508C, at supercritical Relevant to Engineering
conditions the same organics are reformed to gases, for example, Some of the characteristics of SCWO were given by Jain
CO 2 ,H 2 ,CH 4 ,CO 2 , and volatile organic liquids, for example, (1993) are as follows:
alcohols, aldehydes, and furans without producing any char.
Materials as complex as wood can be completely dissolved 1. Supercritical water has high dissolving power, exhi-
and reformed in SCW. These products then can be subjected to biting the characteristics of a nonpolar organic
oxidation; for example, aqueous solutions of organics will solvent (Gloyna and Li, 1995, p. 183). Organic