Page 697 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 697
652 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
compounds dissolve readily in SCW because of its oxygen transport is not an issue, which was a limiting factor
extraordinary solvating powers. Examples of organic in wet-air oxidation (Thornton and Savage, 1992, p. 321).
compounds that may dissolve and be oxidized Companies that have emerged that utilize supercritical oxi-
include polychlorinated biphenols (PCBs), dioxin, dation include Modar, Inc., Natick, Massachusetts; Modec,
benzene, DDT, urea, cyanide, explosives, chemical Inc., Framingham, Massachusetts; Eco-Waste, Inc., Austin,
warfare agents, rocket propellants, chlorophenol, Texas; Air Products and Chemicals, Allentown, Pennyslva-
paint sludge, greases and lubricants, waste oil, nia. The first full-scale reactor was a Texaco SCWO unit
mixed solvents, etc., and ammonia. Most such in Austin, Texas, built in 1995, and which had a capacity
3
compounds are insoluble in ordinary water and are Q ¼ 27.4 m =day (5 gal=min) of organic wastes.
difficult to treat. Also, the solubility of oxygen is
very high. 20.2.3.4 Supercritical Reactors
2. Supercritical water can sustain oxidation. In general, During SCWO, aqueous waste streams are first pressurized
SCFs sustain oxidation reactions because they mix and heated until the water enters the supercritical phase. The
well with nonpolar organic compounds such as oxy- organic components then react in an insulated reactor where
gen, carbon dioxide, methane, and other alkanes. the dissolved components break down further and readily
3. Solubilities of inorganic salts are reduced greatly combine with oxygen; the products approach the ideal sought,
(Li et al., 1993, p. 250) facilitating separation. that is, water and carbon dioxide with about 0.9999 fraction
4. Reaction products of organics and oxygen include conversion expected. With sufficient organic matter in the
carbon dioxide and water, along with ammonia and a feed, that is, 2%–5%, either as the target reactant or as an
variety of low molecular weight acids, mostly acetic ancillary reactant, the reaction is exothermic, that is, self-
acid (Sawicki and Casas, 1993, p. 276). sustaining (Timberlake et al., 1982; Gloyna and Li, 1995;
5. The high specific heat capacity of SCW indicates Modell et al., 1993). For reference, incinerators operate
that it is feasible to hold a high amount of heat within at 20008C < T < 30008C while SCWO reactors operate at
a SCWO reactor (Gloyna and Li, 1995, p. 183). 5008C < T < 6008C (Kruse and Schmieder, 1999, p. 234).
6. With respect to engineering design, (1) high diffu- An engineered supercritical system involves subsystems to
sivities result in high mass-transfer rates, (2) low make the overall system function and to perform economically.
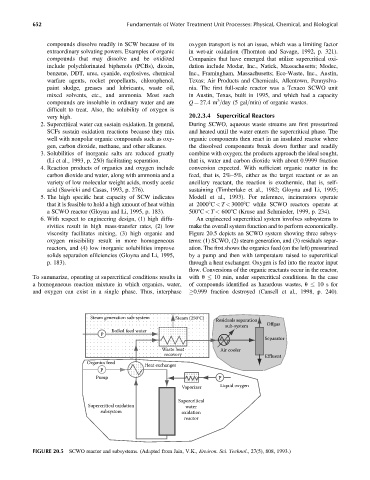
viscosity facilitates mixing, (3) high organic and Figure 20.5 depicts an SCWO system showing three subsys-
oxygen miscibility result in more homogeneous tems: (1) SCWO, (2) steam generation, and (3) residuals separ-
reactors, and (4) low inorganic solubilities improve ation. The first shows the organics feed (on the left) pressurized
solids separation efficiencies (Gloyna and Li, 1995, by a pump and then with temperature raised to supercritical
p. 183). through a heat exchanger. Oxygen is fed into the reactor input
flow. Conversions of the organic reactants occur in the reactor,
To summarize, operating at supercritical conditions results in with u 10 min, under supercritical conditions. In the case
a homogeneous reaction mixture in which organics, water, of compounds identified as hazardous wastes, u 10 s for
and oxygen can exist in a single phase. Thus, interphase 0.999 fraction destroyed (Cansell et al., 1998, p. 240).
Steam generation sub-system Steam (250°C)
Residuals separation
sub-system Offgas
Boiled feed water
P
Separator
Waste heat Air cooler
recovery Effluent
Organics feed
Heat exchanger
P
Pump P
Vaporizer Liquid oxygen
Supercritical
Supercritical oxidation water
subsystem oxidation
reactor
FIGURE 20.5 SCWO reactor and subsystems. (Adapted from Jain, V.K., Environ. Sci. Technol., 27(5), 808, 1993.)