Page 820 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 820
Appendix B: Physical Constants and Physical Data 775
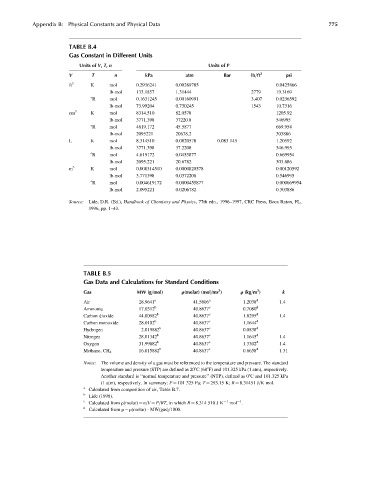
TABLE B.4
Gas Constant in Different Units
Units of V, T, n Units of P
V T n kPa atm Bar lb=ft 2 psi
ft 3 K mol 0.2936241 0.00289785 0.0425866
lb-mol 133.1857 1.31444 2779 19.3169
8R mol 0.1631245 0.00160991 3.407 0.0236592
lb-mol 73.99204 0.730245 1543 10.7316
3
cm K mol 8314.510 82.0578 1205.92
lb-mol 3771.398 37220.8 546995
8R mol 4619.172 45.5877 669.954
lb-mol 2095221 20678.2 303886
L K mol 8.314510 0.0820578 0.083 145 1.20592
lb-mol 3771.398 37.2208 546.995
8R mol 4.619172 0.0455877 0.669954
lb-mol 2095.221 20.6782 303.886
m 3 K mol 0.008314510 0.0000820578 0.00120592
lb-mol 3.771398 0.0372208 0.546995
8R mol 0.004619172 0.0000455877 0.000669954
lb-mol 2.095221 0.0206782 0.303886
Source: Lide, D.R. (Ed.), Handbook of Chemistry and Physics, 77th edn., 1996–1997, CRC Press, Boca Raton, FL,
1996, pp. 1–43.
TABLE B.5
Gas Data and Calculations for Standard Conditions
3
3
Gas MW (g=mol) r(molar) (mol=ms ) r (kg=m ) k
Air 28.9641 a 41.5606 a 1.2038 d 1.4
b c d
Ammonia 17.0312 40.8637 0.7080
Carbon dioxide 44.00982 b 40.8637 c 1.8295 d 1.4
Carbon monoxide 28.0102 b 40.8637 c 1.1644 d
Hydrogen 2.015882 b 40.8637 c 0.0838 d
Nitrogen 28.01342 b 40.8637 c 1.1645 d 1.4
Oxygen 31.99882 b 40.8637 c 1.3302 d 1.4
Methane, CH 4 16.015882 b 40.8637 c 0.6658 d 1.31
Notes: The volume and density of a gas must be referenced to the temperature and pressure. The standard
temperature and pressure (STP) are defined as 208C (688F) and 101.325 kPa (1 atm), respectively.
Another standard is ‘‘normal temperature and pressure’’ (NTP), defined as 08C and 101.325 kPa
(1 atm), respectively. In summary: P ¼ 101 325 Pa; T ¼ 293.15 K; R ¼ 8.31451 J=K mol.
a
Calculated from composition of air, Table B.7.
b
Lide (1998).
c 1 1
Calculated from r(molar) ¼ n=V ¼ P=RT, in which R ¼ 8.314 510 J K mol .
d
Calculated from r ¼ r(molar) MW(gas)=1000.