Page 821 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 821
776 Appendix B: Physical Constants and Physical Data
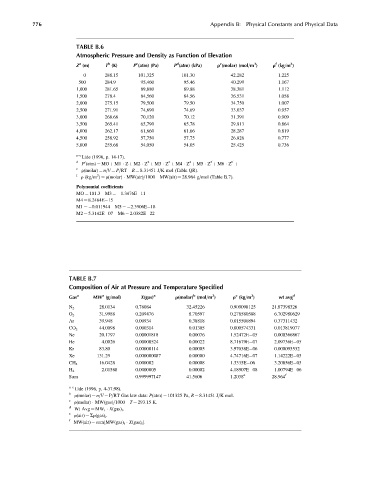
TABLE B.6
Atmospheric Pressure and Density as Function of Elevation
c
b
a
f
3
3
d
e
Z (m) T (K) P (atm) (Pa) P (atm) (kPa) r (molar) (mol=m ) r (kg=m )
0 288.15 101,325 101.30 42.282 1.225
500 284.9 95,460 95.46 40.299 1.167
1,000 281.65 89,880 89.88 38.381 1.112
1,500 278.4 84,560 84.56 36.531 1.058
2,000 275.15 79,500 79.50 34.750 1.007
2,500 271.91 74,690 74.69 33.037 0.957
3,000 268.66 70,120 70.12 31.391 0.909
3,500 265.41 65,790 65.78 29.813 0.864
4,000 262.17 61,660 61.66 28.287 0.819
4,500 258.92 57,750 57.75 26.826 0.777
5,000 255.68 54,050 54.05 25.425 0.736
a–c
Lide (1996, p. 14-17).
d 2 3 4 5 6
0
P (atm) ¼ MO þ M1 Z þ M2 Z þ M3 Z þ M4 Z þ M5 Z þ M6 Z þ
e
r(molar) ¼ n=V ¼ P=RT R ¼ 8.31451 J=K mol (Table QR).
f 3
r (kg=m ) ¼ r(molar) MW(air)=1000 MW(air) ¼ 28.964 g=mol (Table B.7).
Polynomial coefficients
MO ¼ 101.3 M3 ¼ 1.3476E 11
M4 ¼ 8.2464E 15
M1 ¼ 0.011944 M5 ¼ 2.3906E 18
M2 ¼ 5.3142E 07 M6 ¼ 2.0382E 22
TABLE B.7
Composition of Air at Pressure and Temperature Specified
b
a
3
c
3
Gas a MW (g=mol) X(gas) a r(molar) (mol=m ) r (kg=m ) wt avg d
28.0134 0.78084 32.45226 0.909098125 21.87398326
N 2
31.9988 0.209476 8.70597 0.278580588 6.702980629
O 2
Ar 39.948 0.00934 0.38818 0.015506894 0.37311432
44.0098 0.000314 0.01305 0.000574331 0.013819077
CO 2
Ne 20.1797 0.00001818 0.00076 1.52472E 05 0.000366867
He 4.0026 0.00000524 0.00022 8.71679E 07 2.09736E 05
Kr 83.80 0.00000114 0.00005 3.97038E 06 0.000095532
Xe 131.29 0.000000087 0.00000 4.74716E 07 1.14222E 05
CH 4 16.0428 0.000002 0.00008 1.3335E 06 3.20856E 05
2.01588 0.0000005 0.00002 4.18907E 08 1.00794E 06
H 2
Sum 0.999997147 41.5606 1.2038 e 28.964 f
a–c
Lide (1996, p. 4-37:98).
b
r(molar) ¼ n=V ¼ P=RT Gas law data: P(atm) ¼ 101325 Pa, R ¼ 8.31451 J=K mol.
c
r(molar) MW(gas)=1000 T ¼ 293.15 K.
d
Wt Avg ¼ MW i X(gas) i .
e
r(air) ¼ Sr(gas) i .
f
MW(air) ¼ sum[MW(gas) i X(gas) i ].