Page 819 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 819
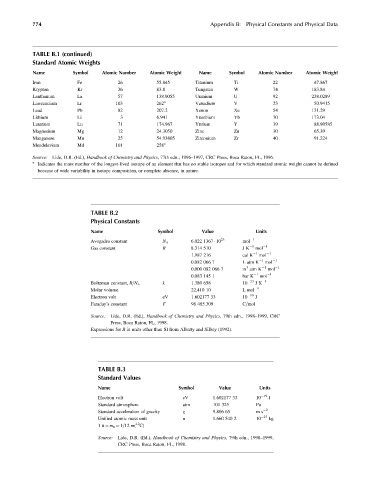
774 Appendix B: Physical Constants and Physical Data
TABLE B.1 (continued)
Standard Atomic Weights
Name Symbol Atomic Number Atomic Weight Name Symbol Atomic Number Atomic Weight
Iron Fe 26 55.845 Titanium Ti 22 47.867
Krypton Kr 36 83.8 Tungsten W 74 183.84
Lanthanum La 57 138.9055 Uranium U 92 238.0289
Lawrencium Lr 103 262 a Vanadium V 23 50.9415
Lead Pb 82 207.2 Xenon Xe 54 131.29
Lithium Li 3 6.941 Ytterbium Yb 70 173.04
Lutetium Lu 71 174.967 Yttrium Y 39 88.90585
Magnesium Mg 12 24.3050 Zinc Zn 30 65.39
Manganese Mn 25 54.93805 Zirconium Zr 40 91.224
Mendelevium Md 101 258 a
Source: Lide, D.R. (Ed.), Handbook of Chemistry and Physics, 77th edn., 1996–1997, CRC Press, Boca Raton, FL, 1996.
a
Indicates the mass number of the longest-lived isotope of an element that has no stable isotopes and for which standard atomic weight cannot be defined
because of wide variability in isotope composition, or complete absence, in nature.
TABLE B.2
Physical Constants
Name Symbol Value Units
Avogadro constant N A 6.022 1367 10 23 mol 1
Gas constant R 8.314 510 J K 1 mol 1
1.987 216 cal K 1 mol 1
0.082 066 7 L atm K 1 mol 1
3
0.000 082 066 7 m atm K 1 mol 1
1 1
0.083 145 1 bar K mol
k 1.380 658 10 23 JK 1
Boltzman constant, R=N A
Molar volume 22.410 10 L mol 1
Electron volt eV 1.602177 33 10 19 J
Faraday’s constant F 96 485.309 C=mol
Source: Lide, D.R. (Ed.), Handbook of Chemistry and Physics, 79th edn., 1998–1999, CRC
Press, Boca Raton, FL, 1998.
Expressions for R in units other than SI from Alberty and Silbey (1992).
TABLE B.3
Standard Values
Name Symbol Value Units
Electron volt eV 1.602177 33 10 19 J
Standard atmosphere atm 101 325 Pa
Standard acceleration of gravity g 9.806 65 m s 2
Unified atomic mass unit u 1.660 540 2 10 27 kg
12
1u ¼ m u ¼ 1=12 m( C)
Source: Lide, D.R. (Ed.), Handbook of Chemistry and Physics, 79th edn., 1998–1999,
CRC Press, Boca Raton, FL, 1998.