Page 888 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 888
Appendix F: Alum Data and Conversions 843
1000
y =M0+M1*x + M2* x 2
900 Compound M0 M1 M2
(SO ) ·14H O –1011.9 312.25 699.2
4 3.
Al 2 4 3 2 Al (SO ) 18H O
2
2
800 Al (SO ) ·18H O –1134.3 350.01 783.01
2
2
4 3
4 3.
2
Al (SO ) –577.37 178 399.11 Al (SO ) 14.3H O
2
4 3
2
700
Alum concentration (g/L) 600 Al –91.909 28.361 63.506 Al (SO )
53.103 118.91
Al O
–172.09
3
2
3+
500
4 3
2
400
300
200 Specified SG(liquid alum) = 1.335 Al O
100 Al 2 3+ 3
0
1.00 1.05 1.10 1.15 1.20 1.25 1.30 1.35 1.40
Specific gravity
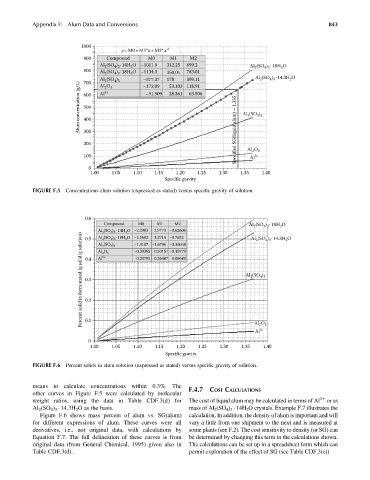
FIGURE F.5 Concentrations alum solution (expressed as stated) versus specific gravity of solution.
0.6
Compound M0 M1 M2 Al (SO ) 18H O
4 3.
2
2
(SO ) · 14H O –2.2903 2.9173 –0.62606
Al 2 2 (SO ) 2 –1.3187 –1.6796 –0.36038 Al 2 (SO ) 14.3H O
4 3
Percent solid in form stated (g solid/g solution) 0.4 Al 3+ –0.20795 0.26487 0.05683 Al 2 (SO 4 ) 3
Al 2 (SO 4 ) 3 · 18H 2 O –2.5682
3.2714 –0.7022
4 3.
0.5
2
4 3
Al 2
–0.39385 0.50151 –0.10779
Al O
3
0.3
0.2
0.1
Al 2 O 3
Al 3+
0
1.00 1.05 1.10 1.15 1.20 1.25 1.30 1.35 1.40
Specific gravity
FIGURE F.6 Percent solids in alum solution (expressed as stated) versus specific gravity of solution.
means to calculate concentrations within 0.3%. The
F.4.7 COST CALCULATIONS
other curves in Figure F.5 were calculated by molecular
weight ratios, using the data in Table CDF.3(d) for The cost of liquid alum may be calculated in terms of Al 3þ or as
Al 2 (SO 4 ) 3 14.3H 2 O as the basis. mass of Al 2 (SO 4 ) 3 14H 2 O crystals. Example F.7 illustrates the
Figure F.6 shows mass percent of alum vs. SG(alum) calculation. In addition, the density of alum is important and will
for different expressions of alum. These curves were all vary a little from one shipment to the next and is measured at
derivatives, i.e., not original data, with calculations by some plants (see F.2). The cost sensitivity to density (or SG) can
Equation F.7. The full delineation of these curves is from be determined by changing this term in the calculations shown.
original data (from General Chemical, 1995) given also in The calculations can be set up in a spreadsheet form which can
Table CDF.3(d). permit exploration of the effect of SG (see Table CDF.3(e))