Page 886 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 886
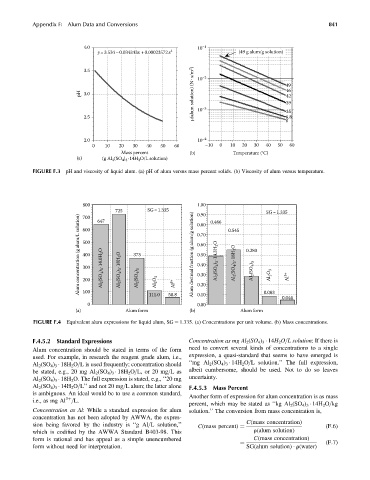
Appendix F: Alum Data and Conversions 841
4.0 10 –1
y = 3.534–0.034243x +0.00023572x 2 (49 g alum/g solution)
3.5 10 –2
46
pH 3.0 μ(alum solution) (N . s/m 2 ) 49
42
39
35
2.5 10 –3 8.8
0
2.0 10 –4
0 10 20 30 40 50 60 –10 0 10 20 30 40 50 60
Mass percent (b) Temperature (°C)
(a) (g AI (SO ) ·14H O/L solution)
2
2
4 3
FIGURE F.3 pH and viscosity of liquid alum. (a) pH of alum versus mass percent solids. (b) Viscosity of alum versus temperature.
800 1.00
725 SG = 1.335 0.90 0.486 0.545 SG= 1.335
Alum concentration (g alum/L solution) 500 Al 2 (SO 4 ) 3 ·14.0H 2 O Al 2 (SO 4 ) 3 ·18H 2 O 373 Alum decimal fraction (g alum/g solution) 0.70 Al 2 (SO 4 ) 3 ·14.3H 2 O Al 2(SO 4) 3 ·18H 2 O 0.280 Al 2 O 3 Al 3+
700
647
0.80
600
0.60
0.50
400
Al 2 (SO 4 ) 3
0.40
300
Al 2 (SO 4 ) 3
0.30
Al 2 O 3
200
100
0.10
0.00
0 111.0 58.8 Al 3+ 0.20 0.083 0.044
(a) Alum form (b) Alum form
FIGURE F.4 Equivalent alum expressions for liquid alum, SG ¼ 1.335. (a) Concentrations per unit volume. (b) Mass concentrations.
F.4.5.2 Standard Expressions Concentration as mg Al 2 (SO 4 ) 3 14H 2 O=L solution: If there is
Alum concentration should be stated in terms of the form need to convert several kinds of concentrations to a single
used. For example, in research the reagent grade alum, i.e., expression, a quasi-standard that seems to have emerged is
Al 2 (SO 4 ) 3 18H 2 O=L is used frequently; concentration should ‘‘mg Al 2 (SO 4 ) 3 14H 2 O=L solution.’’ The full expression,
be stated, e.g., 20 mg Al 2 (SO 4 ) 3 18H 2 O=L, or 20 mg=Las albeit cumbersome, should be used. Not to do so leaves
Al 2 (SO 4 ) 3 18H 2 O. The full expression is stated, e.g., ‘‘20 mg uncertainty.
Al 2 (SO 4 ) 3 14H 2 O=L’’ and not 20 mg=L alum; the latter alone F.4.5.3 Mass Percent
is ambiguous. An ideal would be to use a common standard,
Another form of expression for alum concentration is as mass
i.e., as mg Al =L.
3þ
percent, which may be stated as ‘‘kg Al 2 (SO 4 ) 3 14H 2 O=kg
Concentration as Al: While a standard expression for alum solution.’’ The conversion from mass concentration is,
concentration has not been adopted by AWWA, the expres-
C(mass concentration)
sion being favored by the industry is ‘‘gAl=L solution,’’ (F:6)
C(mass percent) ¼
which is codified by the AWWA Standard B403-98. This r(alum solution)
form is rational and has appeal as a simple unencumbered C(mass concentration)
(F:7)
form without need for interpretation. SG(alum solution) r(water)
¼