Page 900 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 900
Appendix H: Dissolved Gases 855
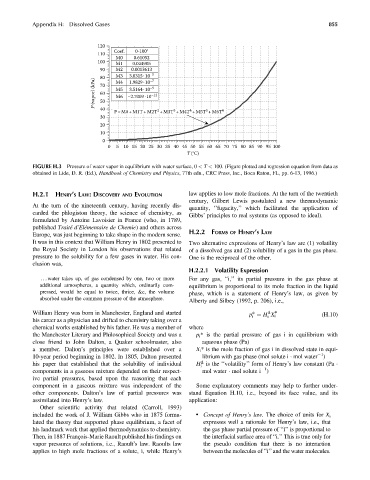
120
Coef. 0-100°
110
M0 0.61052
100 M1 0.044905
90 M2 0.0013613
80 M3 3.0315·10 –5
P (vapor) (kPa) 70 M5 –2.7009· 10 –9
–7
1.9829·10
M4
3.5164·10
60
–12
M6
50
40 2 3 4 5 6
P=M0+M1T+M2T +M3T +M4T +M5T +M6T
30
20
10
0
0 510 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100
T (°C)
FIGURE H.3 Pressure of water vapor in equilibrium with water surface, 0 < T < 100. (Figure plotted and regression equation from data as
obtained in Lide, D. R. (Ed.), Handbook of Chemistry and Physics, 77th edn., CRC Press, Inc., Boca Raton, FL, pp. 6-13, 1996.)
H.2.1 HENRY’S LAW:DISCOVERY AND EVOLUTION law applies to low mole fractions. At the turn of the twentieth
century, Gilbert Lewis postulated a new thermodynamic
At the turn of the nineteenth century, having recently dis-
quantity, ‘‘fugacity,’’ which facilitated the application of
carded the phlogiston theory, the science of chemistry, as
Gibbs’ principles to real systems (as opposed to ideal).
formulated by Antoine Lavoisier in France (who, in 1789,
published Traité d’Elémentaire de Chemie) and others across
Europe, was just beginning to take shape in the modern sense. H.2.2 FORMS OF HENRY’S LAW
It was in this context that William Henry in 1802 presented to Two alternative expressions of Henry’s law are (1) volatility
the Royal Society in London his observations that related of a dissolved gas and (2) solubility of a gas in the gas phase.
pressure to the solubility for a few gases in water. His con- One is the reciprocal of the other.
clusion was,
H.2.2.1 Volatility Expression
. . . water takes up, of gas condensed by one, two or more For any gas, ‘‘i,’’ its partial pressure in the gas phase at
additional atmospheres, a quantity which, ordinarily com- equilibrium is proportional to its mole fraction in the liquid
pressed, would be equal to twice, thrice, &c, the volume phase, which is a statement of Henry’s law, as given by
absorbed under the common pressure of the atmosphere.
Alberty and Silbey (1992, p. 206), i.e.,
William Henry was born in Manchester, England and started D *
p* ¼ H X i (H:10)
i
i
his career as a physician and drifted to chemistry taking over a
chemical works established by his father. He was a member of where
the Manchester Literary and Philosophical Society and was a p i * is the partial pressure of gas i in equilibrium with
close friend to John Dalton, a Quaker schoolmaster, also aqueous phase (Pa)
a member. Dalton’s principles were established over a X i * is the mole fraction of gas i in dissolved state in equi-
1
10-year period beginning in 1802. In 1805, Dalton presented librium with gas phase (mol solute i mol water )
his paper that established that the solubility of individual D
i
H is the ‘‘volatility’’ form of Henry’s law constant (Pa
1
components in a gaseous mixture depended on their respect- mol water mol solute i )
ive partial pressures, based upon the reasoning that each
component in a gaseous mixture was independent of the Some explanatory comments may help to further under-
other components. Dalton’s law of partial pressures was stand Equation H.10, i.e., beyond its face value, and its
assimilated into Henry’s law. application:
Other scientific activity that related (Carroll, 1993)
included the work of J. William Gibbs who in 1875 formu- . ConceptofHenry’slaw. The choice of units for X i
lated the theory that supported phase equilibrium, a facet of expresses well a rationale for Henry’s law, i.e., that
his landmark work that applied thermodynamics to chemistry. the gas phase partial pressure of ‘‘i’’ is proportional to
Then, in 1887 François-Marie Raoult published his findings on the interfacial surface area of ‘‘i.’’ This is true only for
vapor pressures of solutions, i.e., Raoult’s law. Raoults law the pseudo condition that there is no interaction
applies to high mole fractions of a solute, i, while Henry’s between the molecules of ‘‘i’’ and the water molecules.