Page 165 - Gas Purification 5E
P. 165
Alkanolamines for Hydrogen Sulfide and Carbon Dioxide Removal 153
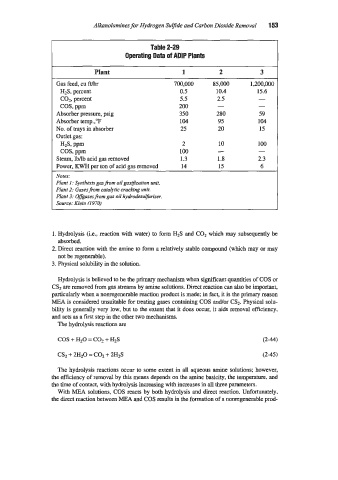
Table 2-29
Operating Data of ADlP Plants
Plant 1 2 3
Gas feed, cu ft/hr 700,000 85,000 1,200,000
H2S, percent 0.5 10.4 15.6
CO?, percent 5.5 2.5 -
COS, ppm 200 - -
Absorber pressure, psig 350 280 59
Absorber temp.,’F 104 95 104
No. of trays in absorber 25 20 15
Outlet gas:
10
HIS, PPm 2 - 100
COS, ppm 100 -
Steam, lbflb acid gas removed 1.3 1.8 2.3
Power, KWH per ton of acid gas removed 14 15 6
.votes:
Plant I: Synthesis gas from oil gasiycation unit.
Plant 2: Gasesfrom catalytic cracking unit.
Plant 3: Offgases from gas oil hydi-odesuljimker.
Source: Klein f1970)
1. Hydrolysis (i.e.: reaction with water) to form HIS and COI which may subsequently be
absorbed.
2. Direct reaction with the amine to form a relatively stable compound (which may or may
not be regenerable).
3. Physical solubility in the solution.
Hydrolysis is believed to be the primary mechanism when significant quantities of COS or
CS2 are removed from gas streams by amine solutions. Direct reaction can also be important,
particularly when a nonregenerable reaction product is made; in fact, it is the primary reason
MEA is considered unsuitable for treating gases containing COS andor CS2. Physical solu-
bility is generally very low, but to the extent that it does occur, it aids removal efficiency.
and acts as a first step in the other two mechanisms.
The hydrolysis reactions are
COS + H20 = CO, + H2S (2-444)
CSI + 2H20 = COZ + 2HzS (2-45)
The hydrolysis reactions occur to some extent in all aqueous amine solutions; however,
the efficiency of removal by this means depends on the amine basicity, the temperature. and
the time of contact, with hydrolysis increasing with increases in all three parameters.
With MEA solutions, COS reacts by both hydrolysis and direct reaction. Unfortunately,
the direct reaction between MEA and COS results in the formation of a nonregenerable prod-