Page 164 - Gas Purification 5E
P. 164
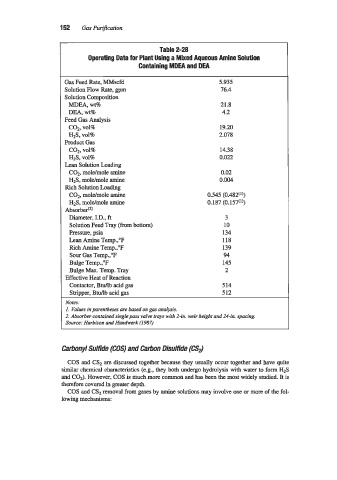
152 Gas Purijkation
Table 2-28
Operating Data for Plant Using a Mixed Aqueous Amine Solution
Containing MDEA and DEA
Gas Feed Rate, MMscfd 5.935
Solution Flow Rate, gpm 76.4
Solution Composition
MDEA, wt% 21.8
DEA, wt% 4.2
Feed Gas Analysis
c02, VO19 19.20
HlS, ~01% 2.078
Product Gas
c02, vol% 14.38
H2S, ~01% 0.022
Lean Solution Loading
COz, moldmole amine 0.02
H2S, mole/mole amine 0.004
Rich Solution Loading
CO?, moldmole amine 0.545 (0.4821'))
H2S, moldmole amine 0.187 (0.157('))
Absorbe@)
Diameter, I.D., ft 3
Solution Feed Tray (from bottom) 10
Pressure, psia 134
Lean Amine Temp.,"F 118
Rich Amine Temp.,"F 139
Sour Gas Temp.,"F 94
Bulge Temp.,"F 145
Bulge Max. Temp. Tray 2
Effective Heat of Reaction
Contactor, Btuflb acid gas 514
Stripper, Bdb acid gas 512
Notes:
1. Values in parentheses are based on gas analysis.
2. rlbsorber contained single pass vahse hap with 2-in. weir height and 24-in. spacing.
Source: Harbison and Handwerk (1987j
Carbonyl Sulfide (COS) and Carbon Disulfide (CSJ
COS and CS2 are discussed together because they usually occur together and have quite
similar chemical characteristics (e.g., they both undergo hydrolysis with water to form H2S
and C02). However, COS is much more common and has been the most widely studied. It is
therefore covered in greater depth.
COS and CSz removal from gases by amine solutions may involve one or more of the fol-
lowing mechanisms: