Page 31 - Gas Purification 5E
P. 31
Introduction 21
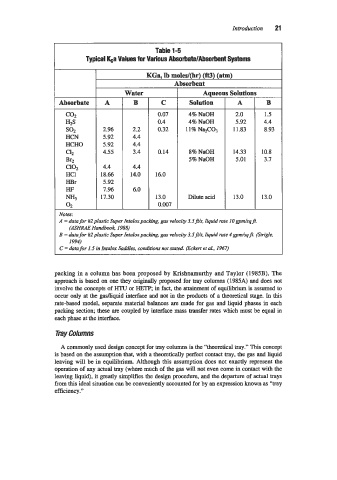
Table 1-5
Typical &a Values for Various AbsorbatdAbsorbent Systems
KGa. lb moles/@r) (ft3) (atm)
bsorbent
Water Aqueo Solutio1
Absorbate A B C Solution A B
co2 0.07 4% NaOH 2.0 1.5
HZS 0.4 4% NaOH 5.92 4.4
so2 2.96 2.2 0.32 11% Na2C03 11.83 8.93
HCN 5.92 4.4
HCHO 5.92 4.4
ClZ 4.55 3.4 0.14 8% NaOH 14.33 10.8
Br2 5% NaOH 5.01 3.7
c102 4.4 4.4
HC1 18.66 14.0 16.0
HBr 5.92
HF 7.96 6.0
NH3 17.30 13.0 Dilute acid 13.0 13.0
02 0.007
Notes:
A = data for #2plastic Super fntaloxpacking, gas velocity 3.5jU.s, liquid rate IOgpdsqff.
(ASHRAE Handbook 1988)
B = data for #2plastic Super Intalax packing, gas velocity 3.5jU.s. liquid rate 4 gpdsqj. (Sirigle,
1994)
C = data for 1.5 in Intalox Saddles, conditions not stated. fickert et al.. 1967)
packing in a column has been proposed by Krishnamurthy and Taylor (1985B). The
approach is based on one they originally proposed for tray columns (1985A) and does not
involve the concepts of HTU or HEW, in fact, the attainment of equilibrium is assumed to
occur only at the gadliquid interface and not in the products of a theoretical stage. In this
rate-based model, separate material balances are made for gas and liquid phases in each
packing section; these are coupled by interface mass transfer rates which must be equal in
each phase at the interface.
Tny Columns
A commonly used design concept for tray columns is the “theoretical tray.” This concept
is based on the assumption that, with a theoretically perfect contact tray, the gas and liquid
leaving will be in equilibrium. Although this assumption does not exactly represent the
operation of any actual tray (where much of the gas will not even come in contact with the
leaving liquid), it greatly simplifies the design procedure, and the departure of actual trays
from this ideal situation can be conveniently accounted for by an expression known as “tray
efficiency.”