Page 32 - Gas Purification 5E
P. 32
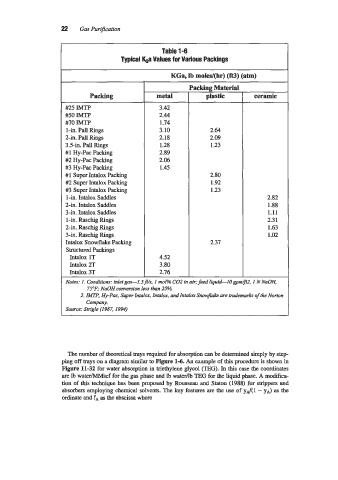
22 Gas Purification
Table 1-6
Typical ka Values for Various Packings
KGa, lb moles/(hr) (fa) (atm)
Packing Material
Packing metal plastic ceramic
#25 IMTP 3.42
#50 IMP 2.44
#70 IMTP 1.74
1-in. Pall Rings 3.10 2.64
2-in. Pall Rings 2.18 2.09
3.5-in. Pall Rings 1.28 1.23
#1 Hy-Pac Packing 2.89
#2 Hy-Pac Packing 2.06
#3 Hy-Pac Packing 1.45
#1 Super Intalox Packing 2.80
#2 Super Intalox Packing 1.92
#3 Super Intalox Packing 1.23
1-in. Intalox Saddles 2.82
2-in. Intalox Saddles 1.88
3-in. Intalox Saddles 1.11
I-in. Raschig Rings 2.3 1
2-in. Raschig Rings 1.63
3-in. Raschig Rings 1.02
Intalox Snowflake Packing 2.37
Structured Packings
Intalox 1T 4.52
Intalox 2T 3.80
Intalox 3T 2.76
Notes: 1. Conditions: inlet ga5-3.5fls. I mol% C02 in air; feed liquid-IO gpdJ2. I NNaOH,
75OF; NaOH conversion less than 25%.
2. IMTP3 Hy-Pac. Super Intalox, Intalox, and Intalar Snowflake are tradenarks of the Norton
Company.
Source: Sirigle (1987, 1994)
The number of theoretical trays required for absorption can be determined simply by step-
ping off trays on a diagram similar to Figure 1-6. An example of this procedure is shown in
Figure 11-32 for water absorption in triethylene glycol (TEG). In this case the coordinates
are lb wakr/MMscf for the gas phase and lb waterflb TEG for the liquid phase. A modifica-
tion of this technique has been proposed by Rousseau and Staton (1988) for strippers and
absorbers employing chemical solvents. The key features are the use of yA/(l - yA) as the
ordinate and fA as the abscissa where