Page 97 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 97
Evaluation Methods and Influencing Factors CHAPTER 2 81
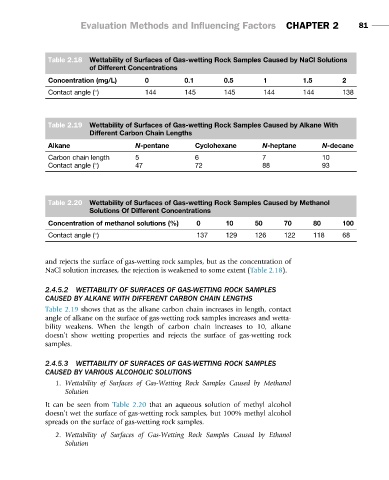
Table 2.18 Wettability of Surfaces of Gas-wetting Rock Samples Caused by NaCl Solutions
of Different Concentrations
Concentration (mg/L) 0 0.1 0.5 1 1.5 2
Contact angle ( ) 144 145 145 144 144 138
Table 2.19 Wettability of Surfaces of Gas-wetting Rock Samples Caused by Alkane With
Different Carbon Chain Lengths
Alkane N-pentane Cyclohexane N-heptane N-decane
Carbon chain length 5 6 7 10
Contact angle ( ) 47 72 88 93
Table 2.20 Wettability of Surfaces of Gas-wetting Rock Samples Caused by Methanol
Solutions Of Different Concentrations
Concentration of methanol solutions (%) 0 10 50 70 80 100
Contact angle ( ) 137 129 126 122 118 68
and rejects the surface of gas-wetting rock samples, but as the concentration of
NaCl solution increases, the rejection is weakened to some extent (Table 2.18).
2.4.5.2 WETTABILITY OF SURFACES OF GAS-WETTING ROCK SAMPLES
CAUSED BY ALKANE WITH DIFFERENT CARBON CHAIN LENGTHS
Table 2.19 shows that as the alkane carbon chain increases in length, contact
angle of alkane on the surface of gas-wetting rock samples increases and wetta-
bility weakens. When the length of carbon chain increases to 10, alkane
doesn’t show wetting properties and rejects the surface of gas-wetting rock
samples.
2.4.5.3 WETTABILITY OF SURFACES OF GAS-WETTING ROCK SAMPLES
CAUSED BY VARIOUS ALCOHOLIC SOLUTIONS
1. Wettability of Surfaces of Gas-Wetting Rock Samples Caused by Methanol
Solution
It can be seen from Table 2.20 that an aqueous solution of methyl alcohol
doesn’t wet the surface of gas-wetting rock samples, but 100% methyl alcohol
spreads on the surface of gas-wetting rock samples.
2. Wettability of Surfaces of Gas-Wetting Rock Samples Caused by Ethanol
Solution