Page 271 - gas transport in porous media
P. 271
268
1.4
1.4 Šolcová and Schneider
He → Ar
H → Ar He → N
2
2
H → N 2 He → H 2
2
p rel 1.2 1.2 p rel
H → He
2
1.0 1.0
1.0 1.0
Ar → N
2
p rel 0.8 Ar → He 0.8 p rel
N → Ar Ar → H
2 2
N → He
2
N → H
2 2
0.6 0.6
0 100 200 0 100 200 300
t (s) t (s)
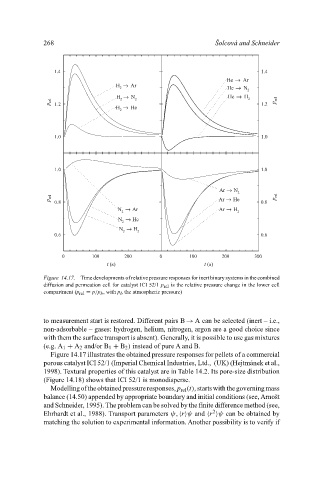
Figure 14.17. Time developments of relative pressure responses for inert binary systems in the combined
diffusion and permeation cell for catalyst ICI 52/1 p rel is the relative pressure change in the lower cell
compartment (p rel = p/p b , with p b the atmospheric pressure)
to measurement start is restored. Different pairs B → A can be selected (inert – i.e.,
non-adsorbable – gases: hydrogen, helium, nitrogen, argon are a good choice since
with them the surface transport is absent). Generally, it is possible to use gas mixtures
(e.g. A 1 + A 2 and/or B 1 + B 2 ) instead of pure A and B.
Figure 14.17 illustrates the obtained pressure responses for pellets of a commercial
porous catalyst ICI 52/1 (Imperial Chemical Industries, Ltd., (UK) (Hejtmánek et al.,
1998). Textural properties of this catalyst are in Table 14.2. Its pore-size distribution
(Figure 14.18) shows that ICI 52/1 is monodisperse.
Modellingoftheobtainedpressureresponses, p rel (t), startswiththegoverningmass
balance (14.50) appended by appropriate boundary and initial conditions (see, Arnošt
and Schneider, 1995). The problem can be solved by the finite difference method (see,
2
Ehrhardt et al., 1988). Transport parameters ψ, r ψ and r ψ can be obtained by
matching the solution to experimental information. Another possibility is to verify if