Page 159 - Geochemical Remote Sensing of The Sub-Surface
P. 159
136 V. T. Jones, M.D. Matthews and D.M. Richers
O(Oa ) 5 DcH4[ppt] (b) 8 13Ccthane[ppt ]
-3 -250 -200 -150 -lO0 -50 -40 -30 -20
-70 -70 t I I I I
/
-60 -60
-50
d
r..) m
cO -40 -40
-30 3~ I
l ~(h~ ~ I
-20
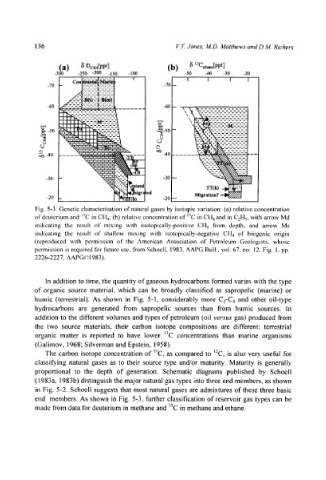
Fig. 5-3. Genetic characterisation of natural gases by isotopic variation: (a) relative concentration
of deuterium and ~3C in C|t4; (b) relative concentration of '3C in CH4 and in C2H5, with arrow Md
indicating the result of mixing with isotopically-positive CH4 from depth, and arrow Ms
indicating the result of shallow mixing with isotopically-negative CH4 ol" biogenic origin
(reproduced with permission of the American Association of Petroleum Geologists, whose
permission is required for future use, from Schoell, 1983, AAPG Bull., vol. 67, no. 12, Fig. 1, pp.
2226-2227, AAPG~) 1983).
In addition to time, the quantity of gaseous hydrocarbons formed varies with the type
of organic source material, which can be broadly classified as sapropelic (marine) or
humic (terrestrial). As shown in Fig. 5-1, considerably more C2-C4 and other oil-type
hydrocarbons are generated from sapropelic sources than from humic sources. In
addition to the different volumes and types of petroleum (oil versus gas) produced from
the two source materials, their carbon isotope compositions are different; terrestrial
organic matter is reported to have lower ~3C concentrations than marine organisms
(Galimov, 1968; Silverman and Epstein, 1958).
The carbon isotope concentration of ~3C, as compared to t2C, is also very useful for
classifying natural gases as to their source type and/or maturity. Maturity is generally
proportional to the depth of generation. Schematic diagrams published by Schoell
(1983a, 1983b) distinguish the major natural gas types into three end members, as shown
in Fig. 5-2. Schoell suggests that most natural gases are admixtures of these three basic
end members. As shown in Fig. 5-3, further classification of reservoir gas types can be
made from data for deuterium in methane and ~3C in methane and ethane.