Page 61 - Geochemical Remote Sensing of The Sub-Surface
P. 61
38 O.F. Putikov and B. Wen
~Cathode
I / / /"l'\ \x
v I t I ~. I i
I I [ I .[ I IOi.][-
V V w v v v v
Me2++2Olff >Me2~(OH)2
0,1T T l T l T l
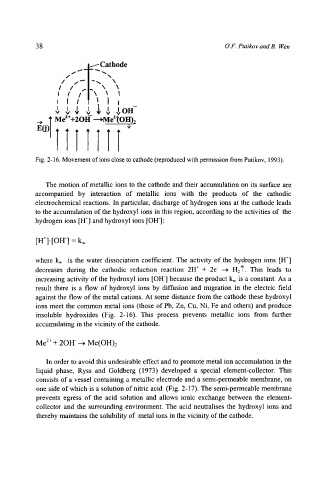
Fig. 2-16. Movement of ions close to cathode (reproduced with permission from Putikov, 1993).
The motion of metallic ions to the cathode and their accumulation on its surface are
accompanied by interaction of metallic ions with the products of the cathodic
electrochemical reactions. In particular, discharge of hydrogen ions at the cathode leads
to the accumulation of the hydroxyl ions in this region, according to the activities of the
hydrogen ions [H +] and hydroxyl ions [OH]:
[H+].[OH -] = kw
where kw is the water dissociation coefficient. The activity of the hydrogen ions [H +]
decreases during the cathodic reduction reaction 2H § + 2e- ---~ H2~. This leads to
increasing activity of the hydroxyl ions [OH] because the product kw is a constant. As a
result there is a flow of hydroxyl ions by diffusion and migration in the electric field
against the flow of the metal cations. At some distance from the cathode these hydroxyl
ions meet the common metal ions (those of Pb, Zn, Cu, Ni, Fe and others) and produce
insoluble hydroxides (Fig. 2-16). This process prevents metallic ions from further
accumulating in the vicinity of the cathode.
Me 2+ + 2OH----~ Me(OH)2
In order to avoid this undesirable effect and to promote metal ion accumulation in the
liquid phase, Ryss and Goldberg (1973) developed a special element-collector. This
consists of a vessel containing a metallic electrode and a semi-permeable membrane, on
one side of which is a solution of nitric acid (Fig. 2-17). The semi-permeable membrane
prevents egress of the acid solution and allows ionic exchange between the element-
collector and the surrounding environment. The acid neutralises the hydroxyl ions and
thereby maintains the solubility of metal ions in the vicinity of the cathode.