Page 62 - Geochemical Remote Sensing of The Sub-Surface
P. 62
Geoelectrochemistry and stream dispersion 39
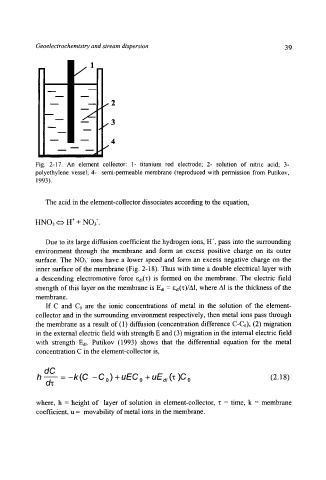
Fig. 2-17. An element collector: l- titanium rod electrode; 2- solution of nitric acid; 3-
polyethylene vessel; 4- semi-permeable membrane (reproduced with permission from Putikov,
1993).
The acid in the element-collector dissociates according to the equation,
HNO3 ~ H + + NO3.
Due to its large diffusion coefficient the hydrogen ions, H +, pass into the surrounding
environment through the membrane and form an excess positive charge on its outer
surface. The NO3 ions have a lower speed and form an excess negative charge on the
inner surface of the membrane (Fig. 2-18). Thus with time a double electrical layer with
a descending electromotive force gd~(~) is formed on the membrane. The electric field
strength of this layer on the membrane is Edl = gd~(~)/A1, where AI is the thickness of the
membrane.
If C and Co are the ionic concentrations of metal in the solution of the element-
collector and in the surrounding environment respectively, then metal ions pass through
the membrane as a result of (1) diffusion (concentration difference C-C0), (2) migration
in the external electric field with strength E and (3) migration in the internal electric field
with strength Ed~. Putikov (1993) shows that the differential equation for the metal
concentration C in the element-collector is,
dC
h dr - -k (C - C o) + uEC o + uEa~ ('r.)C o (2.18)
where, h = height of layer of solution in element-collector, 9 = time, k = membrane
coefficient, u = movability of metal ions in the membrane.