Page 60 - Geochemical Remote Sensing of The Sub-Surface
P. 60
Geoelectrochemistry and stream dispersion 37
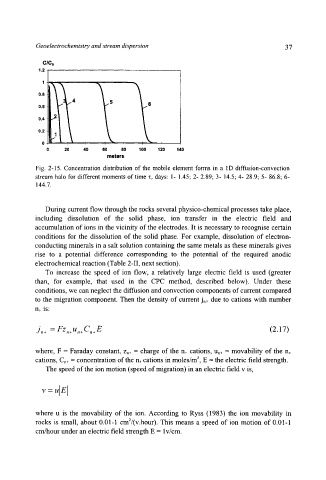
ClCo
1.2
0.8
5 6
0.6
0.4
0.2
t
0 20 40 60 80 100 120 140
meters
Fig. 2-15. Concentration distribution of the mobile element forms in a l D diffusion-convection
stream halo for different moments of time x, days: 1- 1.45 2- 2.89; 3- 14.5; 4- 28.9; 5- 86.8" 6-
144.7.
During current flow through the rocks several physico-chemical processes take place,
including dissolution of the solid phase, ion transfer in the electric field and
accumulation of ions in the vicinity of the electrodes. It is necessary to recognise certain
conditions for the dissolution of the solid phase. For example, dissolution of electron-
conducting minerals in a salt solution containing the same metals as these minerals gives
rise to a potential difference corresponding to the potential of the required anodic
electrochemical reaction (Table 2-II, next section).
To increase the speed of ion flow, a relatively large electric field is used (greater
than, for example, that used in the CPC method, described below). Under these
conditions, we can neglect the diffusion and convection components of current compared
to the migration component. Then the density of current jn+ due to cations with number
n+ is:
j,+ - Fz ,+ u ,+ C,+ E (2.17)
where, F = Faraday constant, z,+ = charge of the n+ cations, u.+ = movability of the n+
cations, C,+ = concentration of the n+ cations in moles/m 3, E = the electric field strength.
The speed of the ion motion (speed of migration) in an electric field v is,
- u)EI
where u is the movability of the ion. According to Ryss (1983) the ion movability in
rocks is small, about 0.01-1 cm/(v.hour). This means a speed of ion motion of 0.01-1
2
cm/hour under an electric field strength E = 1 v/cm.