Page 55 - Geochemical Remote Sensing of The Sub-Surface
P. 55
32 O.F. Putikov and B. Wen
u, pg/I
100000
10000
1000
100
3
10
J J I
0 50 100 150 200 250
mm
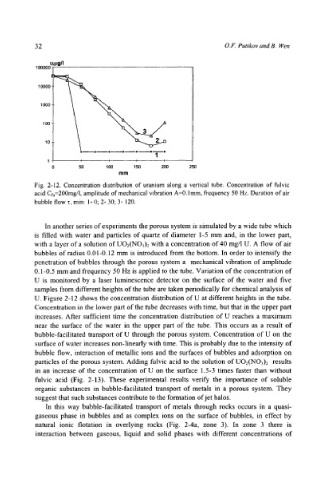
Fig. 2-12. Concentration distribution of uranium along a vertical tube. Concentration of fulvic
acid Cfa=200mg/l, amplitude of mechanical vibration A=0. l mm, frequency 50 Hz. Duration of air
bubble flow x, min: 1- 0; 2- 30; 3- 120.
In another series of experiments the porous system is simulated by a wide tube which
is filled with water and particles of quartz of diameter 1-5 mm and, in the lower part,
with a layer of a solution of UO2(NO3)2 with a concentration of 40 mg/1 U. A flow of air
bubbles of radius 0.01-0.12 mm is introduced from the bottom. In order to intensify the
penetration of bubbles through the porous system a mechanical vibration of amplitude
0.1-0.5 mm and frequency 50 Hz is applied to the tube. Variation of the concentration of
U is monitored by a laser luminescence detector on the surface of the water and five
samples from different heights of the tube are taken periodically for chemical analysis of
U. Figure 2-12 shows the concentration distribution of U at different heights in the tube.
Concentration in the lower part of the tube decreases with time, but that in the upper part
increases. After sufficient time the concentration distribution of U reaches a maximum
near the surface of the water in the upper part of the tube. This occurs as a result of
bubble-facilitated transport of U through the porous system. Concentration of U on the
surface of water increases non-linearly with time. This is probably due to the intensity of
bubble flow, interaction of metallic ions and the surfaces of bubbles and adsorption on
particles of the porous system. Adding fulvic acid to the solution of UO2(NO3)2 results
in an increase of the concentration of U on the surface 1.5-3 times faster than without
fulvic acid (Fig. 2-13). These experimental results verify the importance of soluble
organic substances in bubble-facilitated transport of metals in a porous system. They
suggest that such substances contribute to the formation of jet halos.
In this way bubble-facilitated transport of metals through rocks occurs in a quasi-
gaseous phase in bubbles and as complex ions on the surface of bubbles, in effect by
natural ionic flotation in overlying rocks (Fig. 2-4a, zone 3). In zone 3 there is
interaction between gaseous, liquid and solid phases with different concentrations of