Page 52 - Geochemical Remote Sensing of The Sub-Surface
P. 52
Geoelectrochemistry and stream dispersion 29
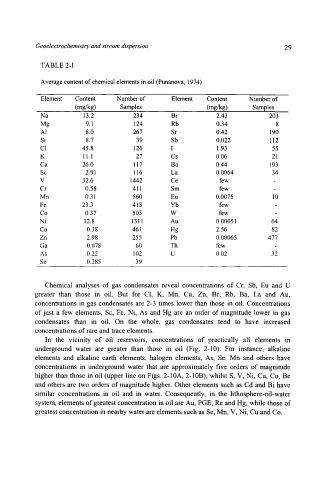
TABLE 2-I
Average content of chemical elements in oil (Punanova, 1974)
Element Content Number of Element Content Number of
(mg/kg) Samples (mg/kg) Samples
Na 13.2 234 Br 2.43 203
Mg 9.1 124 Rb 0.34 8
AI 8.0 267 Sr 0.42 190
Si 8.7 39 Sb 0.022 112
C1 45.8 126 I 1.93 55
K 11.1 27 Cs 0.06 21
Ca 26.0 117 Ba 0.44 193
Sc 2.91 116 La 0.0064 34
V 32.6 1442 Ce few -
Cr 0.58 411 Sm few -
Mn 0.31 560 Eu 0.0075 10
Fe 23.3 418 Yb few -
Co 0.37 503 W few -
Ni 12.8 1311 Au 0.00051 64
Cu 0.38 461 Hg 2.56 82
Zn 2.98 255 Pb 0.00065 477
Ga 0.078 60 Th few -
As 0.22 102 U 0.02 32
Se 0.285 39
Chemical analyses of gas condensates reveal concentrations of Cr, Sb, Eu and U
greater than those in oil. But for C1, K, Mn, Cu, Zn, Br, Rb, Ba, La and Au,
concentrations in gas condensates are 2-3 times lower than those in oil. Concentrations
of just a few elements, Sc, Fe, Ni, As and Hg are an order of magnitude lower in gas
condensates than in oil. On the whole, gas condensates tend to have increased
concentrations of rare and trace elements.
In the vicinity of oil reservoirs, concentrations of practically all elements in
underground water are greater than those in oil (Fig. 2-10). For instance, alkaline
elements and alkaline earth elements, halogen elements, As, Se, Mn and others have
concentrations in underground water that are approximately five orders of magnitude
higher than those in oil (upper line on Figs. 2-10A, 2-10B), whilst S, V, Ni, Cu, Co, Be
and others are two orders of magnitude higher. Other elements such as Cd and Bi have
similar concentrations in oil and in water. Consequently, in the lithosphere-oil-water
system, elements of greatest concentration in oil are Au, PGE, Re and Hg, while those of
greatest concentration in nearby water are elements such as Se, Mn, V, Ni, Cu and Co.