Page 157 - Geochemistry of Oil Field Waters
P. 157
STRONTIUM 145
&-\- Normal evaporite curve
M
P
P
Ii M
M
201 / 1
00
CALCIUM, mg/ I
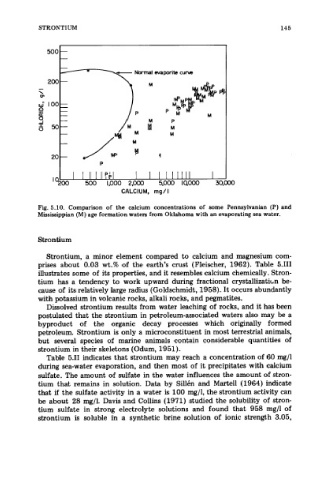
Fig. 5.10. Comparison of the calcium concentrations of some Pennsylvanian (P) and
MEsissippian (M) age formation waters from Oklahoma with an evaporating sea water.
Strontium
Strontium, a minor element compared to calcium and magnesium com-
prises about 0.03 wt.% of the earth's crust (Fleischer, 1962). Table 5.111
illustrates some of its properties, and it resembles calcium chemically. Stron-
tium has a tendency to work upward during fractional crystallizaticn be-
cause of its relatively large radius (Goldschmidt, 1958). It occurs abundantly
with potassium in volcanic rocks, alkali rocks, and pegmatites.
Dissolved strontium results from water leaching of rocks, and it has been
postulated that the strontium in petroleum-associated waters also may be a
byproduct of the organic decay processes which originally formed
petroleum. Strontium is only a microconstituent in most terrestrial animals,
but several species of marine animals contain considerable quantities of
strontium in their skeletons (Odum, 1951).
Table 5.11 indicates that strontium may reach a concentration of 60 mg/l
during sea-water evaporation, and then most of it precipitates with calcium
sulfate. The amount of sulfate in the water influences the amount of stron-
tium that remains in solution. Data by Sillhn and Martell (1964) indicate
that if the sulfate activity in a water is 100 mg/l, the strontium activity can
be about 28 mg/l. Davis and Collins (1971) studied the solubility of stron-
tium sulfate in strong electrolyte solutions and found that 958 mg/l of
strontium is soluble in a synthetic brine solution of ionic strength 3.05,