Page 156 - Geochemistry of Oil Field Waters
P. 156
144 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
but only in the crust; in the earth as a whole, magnesium is much more
abundant. Calcium is dissolved as bicarbonate as a result of chemical
weathering of calcium-bearing minerals. Waters associated with limestone,
dolomite, gypsum, or gypsiferous shale usually contain an abundance of
calcium, but waters associated with granite or silicious sand may contain less
than 10 mg/l of calcium. Slight changes in the pH of waters containing
calcium bicarbonate will cause calcium carbonate to precipitate, and calcium
carbonate is one of the most common deposits found in plugged oilfield
lines, equipment, and reservoirs.
Precipitation of calcium carbonate in the sea is the prime mode of the
origin of limestone. The solubility of calcium carbonate in sea water in-
creases with salinity and increasing partial pressure of carbon dioxide, but it
decreases with increasing pH, calcium content, and temperature. The
solubility of calcium sulfate decreases with increasing temperature.
Shales, sandstones, and carbonate rocks contain about 22,100, 39,100,
and 302,300 ppm of calcium, respectively (Mason, 1966). Sea water contains
400 mg/l and subsurface brines often contain 2,000-3,000 mg/l, with some
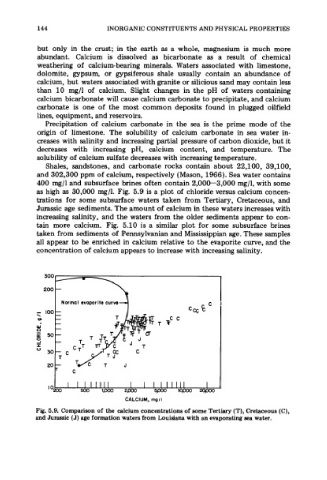
as high as 30,000 mg/l. Fig. 5.9 is a plot of chloride versus calcium concen-
trations for some subsurface waters taken from Tertiary, Cretaceous, and
Jurassic age sediments. The amount of calcium in these waters increases with
increasing salinity, and the waters from the older sediments appear to con-
tain more calcium. Fig. 5.10 is a similar plot for some subsurface brines
taken from sediments of Pennsylvanian and Mississippian age. These samples
all appear to be enriched in calcium relative to the evaporite curve, and the
concentration of calcium appears to increase with increasing salinity.
-
200
Normal evaporite curve
- 100- -
\ -
0
1 I 1 I I I111
500 1,m 2 p 5poo lop00 29ooo
CALCIUM, mg/l
Fig. 5.9. Comparison of the calcium concentrations of some Tertiary (T), Cretaceous (C),
and Jurassic (J) age formation waters from Louisiana with an evaporating sea water.