Page 153 - Geochemistry of Oil Field Waters
P. 153
CESIUM 141
Cesium
Cesium is the heaviest alkali metal and also the rarest, with an abundance
of about 7 x wt.% in the earth’s crust (Fleischer, 1962). It has an ionic
radius of 1.69 8, which is distinctly larger than potassium, and it cannot
replace potassium in minerals as easily as rubidium; probably because of this,
it forms its own minerals. It is leached from igneous and metamorphic rocks
by water during weathering, and is adsorbed by hydrolysate sediments and
soils more readily than rubidium or potassium. Its low ionization potential
indicates that it has the greatest chemical reactivity of the alkali metals.
Cesium and rubidium were discovered in 1860 by Robert Bunsen by use of
spectral analysis, a method which he and Kirchhoff invented.
Cesium concentrates primarily like rubidium, in marine argillaceous sedi-
ments. Some shales contain about 15 ppm; deep-sea red clays, 20 ppm; and
glauconite, 15 ppm of cesium (Goldschmidt, 1958). Sea water contains
5 x mg/l of cesium, and some subsurface brines contain up to 1 mg/l.
Beryllium
Beryllium is a member of the alkaline earth group in the periodic chart of
the elements, but few of its properties are similar to the more abundant
members, such as magnesium, calcium, and strontium. Beryllium, like
lithium, is a light element with an atomic weight of 9.012 (Table 5.111; see
also Moeller, 1954), and like lithium, it is an exception to the rule that light
elements are more abundant than heavy elements. The earth’s crust contains
about 6 x wt.% of beryllium (Fleischer, 1962).
In sedimentary rocks, beryllium is restricted primarily to hydrolysates and
especially to bauxites enriched in aluminum (Goldschmidt, 1958). Shales
contain about 6 ppm, and some coal ashes contain up to 8,000 ppm, al-
though generally only about 4 ppm. The concentration of beryllium in sea
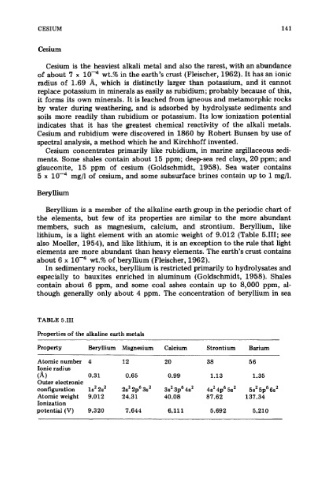
TABLE 5.111
Properties of the alkaline earth metals
Property Beryllium Magnesium Calcium Strontium Barium
Atomic number 4 12 20 38 56
Ionic radius
(A 1 0.31 0.65 0.99 1.13 1.35
Outer electronic
configuration 1s’ 2s’ 2s’ 2p6 3s’ 3s2 3p6 4s2 4s’ 4p6 5s2 5s2 5p6 6s’
Atomic weight 9.012 24.31 40.08 87.62 137.34
Ionization
potential (V) 9.320 7.644 6.111 5.692 5.210