Page 151 - Geochemistry of Oil Field Waters
P. 151
POTASSIUM 139
.lvv ~~
-
200
-
- 100
1 I I I I Ill
POTASSIUM, g / I
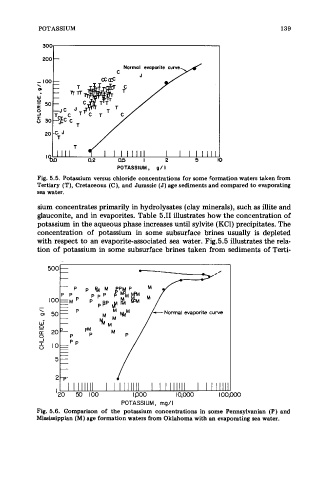
Fig. 5.5. Potassium versus chloride concentrations for some formation waters taken from
Tertiary (T), Cretaceous (C), and Jurassic (J) age sediments and compared to evaporating
sea water.
sium concentrates primarily in hydrolysates (clay minerals), such as illite and
glauconite, and in evaporites. Table 5.11 illustrates how the concentration of
potassium in the aqueous phase increases until sylvite (KC1) precipitates. The
concentration of potassium in some subsurface brines usually is depleted
with respect to an evaporite-associated sea water. Fig.5.5 illustrates the rela-
tion of potassium in some subsurface brines taken from sediments of Terti-
500 - ,'
-
-
-Nmal evaporite curve
-
-
5-
-
-
m
POTASSIUM, mg/l
Fig. 5.6. Comparison of the potassium concentrations in some Pennsylvanian (P) and
Mississippian (M) age formation waters from Oklahoma with an evaporating sea water.