Page 146 - Geochemistry of Oil Field Waters
P. 146
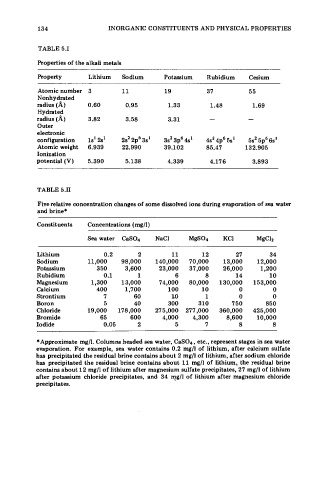
134 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
TABLE 5.1
Properties of the alkali metals
Property Lithium Sodium Potassium Rubidium Cesium
Atomic number 3 11 19 37 55
Nonhydrated
radius (A) 0.60 0.95 1.33 1.48 1.69
Hydrated
radius (A) 3.82 3.58 3.31 - -
Outer
electronic
configuration 1s' 2s' 2s2 2p6 3s' 3s' 3p6 4s' 4s2 4p6 5s' 5s' 5p6 6s'
Atomic weight 6.939 22.990 39.102 85.47 132.905
Ionization
potential (V) 5.390 5.138 4.339 4.176 3.893
TABLE 5.11
Five relative concentration changes of some dissolved ions during evaporation of sea water
and brine*
Constituents Concentrations (mg/l)
Sea water CaSO4 NaCl MgS04 KCI MgC12
Lithium 0.2 2 11 12 27 34
Sodium 11,000 98,000 140,000 70,000 13,000 12,000
Potassium 350 3,600 23,000 37,000 26,000 1,200
Rubidium 0.1 1 6 8 14 10
Magnesium 1,300 13,000 74,000 80,000 130,000 153,000
Calcium 400 1,700 100 10 0 0
Strontium 7 60 10 1 0 0
Boron 5 40 300 310 750 850
Chloride 19,000 178,000 275,000 277,000 360,000 425,000
Bromide 65 600 4,000 4,300 8,600 10,000
Iodide 0.05 2 5 7 8 8
*Approximate mg/l. Columns headed sea water, CaS04, etc., represent stages in sea water
evaporation. For example, sea water contains 0.2 mg/l of lithium, after calcium sulfate
has precipitated the residual brine contains about 2 mg/l of lithium, after sodium chloride
has precipitated the residual brine contains about 11 mg/l of lithium, the residual brine
contains about 12 mg/l of lithium after magnesium sulfate precipitates, 27 mg/l of lithium
after potassium chloride precipitates, and 34 mg/l of lithium after magnesium chloride
precipitates.