Page 147 - Geochemistry of Oil Field Waters
P. 147
LITHIUM 135
The hydrated radius of lithium is 3.82 a, as shown in Table 5.1 (Moeller,
1954). The ionic potential is 0.60, and the polarization is 1.67. The polariza-
tion is quite high and is a measure of its replacing power in an exchange
system. Apparently it can replace strontium, calcium, and magnesium since
their polarizations are 1.77, 2.02, and 3.08, respectively.
Some surface waters of the volcanic sodium chloride type are enriched in
lithium (White, 1957). Lithium from Searles Lake brine is recovered as
Li2NaP04 (Brasted, 1957). The content of lithium in oilfield waters is
usually less than 10 mg/l but in some Smackover formation waters from east
Texas, concentrations up to 500 mg/l are present. When a brine containing
lithium goes through an evaporite sequence, lithium is one of the elements
whose concentration does not decrease, as illustrated in Table 5.11, in the
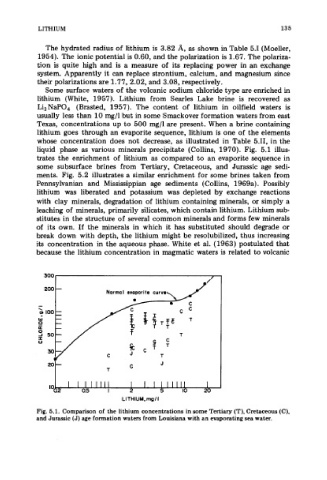
liquid phase as various minerals precipitate (Collins, 1970). Fig. 5.1 illus-
trates the enrichment of lithium as compared to an evaporite sequence in
some subsurface brines from Tertiary, Cretaceous, and Jurassic age sedi-
ments. Fig. 5.2 illustrates a similar enrichment for some brines taken from
Pennsylvanian and Mississippian age sediments (Collins, 1969a). Possibly
lithium was liberated and potassium was depleted by exchange reactions
with clay minerals, degradation of lithium containing minerals, or simply a
leaching of minerals, primarily silicates, which contain lithium. Lithium sub-
stitutes in the structure of several common minerals and forms few minerals
of its own. If the minerals in which it has substituted should degrade or
break down with depth, the lithium might be resolubilized, thus increasing
its concentration in the aqueous phase. White et al. (1963) postulated that
because the lithium concentration in magmatic waters is related to volcanic
LITHIUM, mgll
Fig. 5.1. Comparison of the lithium concentrations in some Tertiary (T), Cretaceous (C),
and Jurassic (J) age formation waters from Louisiana with an evaporating sea water.