Page 148 - Geochemistry of Oil Field Waters
P. 148
136 INORGANIC CONSTITUENTS AND PHYSICAL PROPERTIES
u
10 20 3
LITHIUM, mg/l
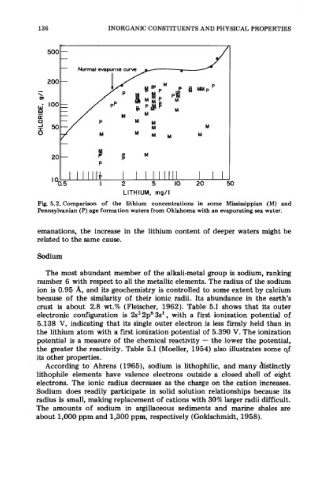
Fig. 5.2. Comparison of the lithium concentrations in some Mississippian (M) and
Pennsylvanian (P) age formation waters from Oklahoma with an evaporating sea water.
emanations, the increase in the lithium content of deeper waters might be
related to the same cause.
Sodium
The most abundant member of the alkali-metal group is sodium, ranking
number 6 with respect to all the metallic elements. The radius of the sodium
ion is 0.95 A, and its geochemistry is controlled to some extent by calcium
because of the similarity of their ionic radii. Its abundance in the earth's
crust is about 2.8 wt.% (Fleischer, 1962). Table 5.1 shows that its outer
electronic configuration is 2s' 2p6 3s' , with a first ionization potential of
5.138 V, indicating that its single outer electron is less firmly held than in
the lithium atom with a first ionization potential of 5.390 V. The ionization
potential is a measure of the chemical reactivity - the lower the potential,
the greater the reactivity. Table 5.1 (Moeller, 1954) also illustrates some qf
its other properties.
According to Ahrens (1965), sodium is lithophilic, and many distinctly
lithophile elements have valence electrons outside a closed shell of eight
electrons. The ionic radius decreases as the charge on the cation increases.
Sodium does readily participate in solid solution relationships because its
radius is small, making replacement of cations with 30% larger radii difficult.
The amounts of sodium in argillaceous sediments and marine shales are
about 1,000 ppm and 1,300 ppm, respectively (Goldschmidt, 1958).